Canada’s Foodborne Illness Outbreak Response Protocol (FIORP): A guide to multi-jurisdictional enteric outbreak response
Table of Contents
- 1. Definitions
- 2. Introduction
- 3. Purpose
- 4. Scope
- 5. Guiding principles
- 6. Roles and responsibilities
- 7. Operating procedures
- 7.1 Identification of a potential multi-jurisdictional foodborne illness outbreak
- 7.2 Notification of partners
- 7.3 OICC assessment call and OICC Activation
- 7.4 Outbreak Investigation Coordinating Committee (OICC)
- 7.5 Coordinated investigations
- 7.6 Centralized integrated analysis
- 7.7 Health risk assessment
- 7.8 Public health and food safety actions
- 7.9 Communication with the public
- 7.10 OICC deactivation and outbreak conclusion
- 7.11 Post outbreak debrief
- 8. Administrative review
- 9. Emergency operations centre activation and incident command system
- 10. List of acronyms
- Annexes
- Annex 1 – Outbreak Investigation Coordinating Committee teleconference agenda template
- Annex 2 – Canadian Network for Public Health Intelligence (CNPHI)
- Annex 3 – Assessment of the weight of epidemiological evidence
- Annex 4 – Botulism reference service for Canada
- Annex 5 – Listeriosis reference service for Canada
- Annex 6 – Food virology reference centre for Canada
- Annex 7 – Canadian Food Inspection Agency - Laboratory testing
- Annex 8 – Surveillance for foodborne illness
- Annex 9 – Laboratory capability for food and clinical microbiology
- Annex 10 – Communicating with the public and those at greater risk: tactics and evaluation
- Annex 11 – Template for outbreak debrief/review
- Annex 12 – F/P/T detailed roles and responsibilities
- Annex 13 – Standard Operating Procedure (SOP) for directing food samples collected by provincial/territorial/municipal inspectors during epidemiological/public health/food safety investigation to the Federal Laboratory Network
- Annex 14 – Multi-jurisdictional Enteric Illness Outbreak investigations linked to contact with animals or animal foods

Download the alternative format
(PDF format, 2,632 KB, 120 pages)
Preamble
The investigation of and response to multi-jurisdictional foodborne illness outbreaks in Canada involves several organizations at multiple levels of government with complementary responsibilities. The Foodborne Illness Outbreak Response Protocol (FIORP) was collectively developed by the Public Health Agency of Canada (PHAC), Health Canada (HC), and the Canadian Food Inspection Agency (CFIA), in consultation with provincial and territorial (P/T) stakeholders, to enhance the collaboration and overall effectiveness of response during multi-jurisdictional foodborne illness outbreaks.
The first edition of the FIORP was developed in 1999 by HC and the CFIA, in consultation with the P/Ts. In 2004, the protocol was endorsed by the former Federal/Provincial/Territorial (F/P/T) Committee on Food Safety Policy, the Council of Chief Medical Officers of Health (CCMOH), and the F/P/T Deputy Ministers of Health. Following the 2008 Listeriosis outbreak, the FIORP was updated in 2010 with F/P/T input and a commitment to repeat the formal review process every five years. The current version was developed after consultation with F/P/T stakeholders throughout 2014 and 2015 and received endorsement by the F/P/T Deputy Ministers of Health and Agriculture and Agri-Food, the CCMOH, and the Public Health Network Council.
The contributions of all the individuals who participated in the revision and consultation process are gratefully appreciated.
For more information on or to receive a copy of the FIORP please contact the Centre for Foodborne, Environmental and Zoonotic Infectious Diseases (CFEZID) at PHAC by e-mail: fiorp.mitioa@phac-aspc.gc.ca
1. Definitions
The following definitions are provided to establish a common understanding of the terms in this document.
Centralized Integrated Analysis: When multiple agencies are involved, centralized collation and analysis of data by the Outbreak Investigation Coordinating Committee (OICC) lead agency is required to inform decision-making and draw conclusions based on all available data.
Cluster: An unusual aggregation of similar health events, generally grouped together as they appear over a particular time period or geographical area. A cluster may be seen as the occurrence of cases of disease (human illnesses) in excess of what is usually expected for a given period of time. A cluster may or may not reach the status of an “outbreak.”
Emergency Operations Centre (EOC): The physical location where an organization comes together during an emergency to coordinate response and recovery actions, and resources. These centres may alternatively be called command centres, situation rooms, war rooms, crisis management centres, or other similar terms. Regardless of the term, this is where the coordination of information and resources takes place. The EOC is not an incident command post; rather, it is the operations centre where coordination and management decisions are facilitated.
Enteric illness: A disease of the gastrointestinal tract caused by an infection or intoxication resulting from the ingestion of bacteria, viruses, parasites, or toxins transmitted through food, water, animals or person-to-person contact.
Epidemiological investigation: Investigation made to determine the existence of an outbreak; to characterize it over a specific time period, geographical area and describe personal characteristics of cases; and to develop and test a hypothesis explaining the specific exposure that caused disease. The investigation may result in recommendations towards the implementation of appropriate prevention and mitigation measures.
Epidemiological evidence: The demonstration of an association between a source of exposure and human illness.
Evidence: That which demonstrates or shows an association between a source of exposure and an illness. Evidence of an association between a consumed food and human illness may be epidemiological and/or based on the results of food safety investigations or laboratory analysis.
FIORP duty officer: The primary representative(s) within an organization who is responsible for briefing senior officials and ensuring that his or her organization leads or participates in an OICC as required.
Food: Includes any article manufactured, sold or represented for use as food or drink for human beings, chewing gum, and any ingredient that may be mixed with food for any purpose whatever.
Foodborne hazard: A biological, chemical, or physical agent in food, or a condition of food, that has the potential to cause an adverse health effect.
Foodborne illness: A human illness, with evidence indicating a food was the source of exposure to the contaminant causing illness. Foodborne illness occurs when a person consumes food contaminated with a biological or chemical hazard.
Foodborne injury: Damage to the human gastrointestinal tract resulting from consumption of a food contaminated with physical hazards. Common types of foodborne injuries include cuts, bleeding, choking and broken teeth.
Food safety investigation: Inspection and related activities undertaken by regulatory officials to verify whether or not a food hazard that could cause human illness exists and to determine the nature and extent of the problem.
Health Risk Assessment (HRA): A scientifically based process to determine the likelihood that a specific adverse health effect will occur in an individual or a population following exposure to a hazardous agent. The following steps are used in the development of a health risk assessment: 1) hazard identification, 2) hazard characterization, 3) exposure assessment, and 4) risk characterization.
Incident Command System (ICS): A standardized on-scene emergency management concept specifically designed to allow its user(s) to adopt an integrated organizational structure equal to the complexity and demands of single or multiple incidents, without being hindered by jurisdictional boundaries.
Laboratory evidence: The demonstration of an association between cases of human illness, or between cases of human illness and the suspect source, through the isolation/identification of the same pathogen, toxin, or contaminant from both sources.
Multi-jurisdictional foodborne illness outbreak: A foodborne illness outbreak that occurs in more than one P/T or occurs in Canada and involves another country or countries and requires the resources of more than one F/P/T public health and/or food regulatory organization to investigate or control it.
Outbreak: An incident in which two or more persons experience similar illness and there is epidemiologic evidence of an association between them.
Partner: Any agency with a responsibility to investigate or respond to foodborne illness outbreaks in Canada, including F/P/T health and agriculture and agri-food agencies that share food safety and public health responsibilities.
Recall: A firm to remove from further sale or use, or to correct, a marketed product that poses a risk and/or contravenes a legislation administered or enforced by a regulatory authority
Response: In the context of foodborne illness outbreaks, response includes activities related to the determination, investigation, mitigation, and containment of such outbreaks, as well as related communication activities.
Stakeholder: Any organization, group, or person who can be affected by foodborne illness outbreak investigations in Canada. This can include government agencies, industry organizations, health care system, media, and the public.
2. Introduction
Foodborne illness or injury results from the natural, accidental, or malicious contamination of foods by biological, chemical, or physical hazards. The impacts of foodborne illness may include morbidity and mortality, increased health care costs, loss of consumer confidence, economic losses, and lost productivity to industry.
The globalization of our food supply has resulted in large volumes of raw and processed products moving across domestic and international boundaries every day. Consequently, foodborne illness outbreaks associated with widely distributed contaminated foods result in human illnesses that cross local, P/T and national boundaries.
Regulatory bodies responsible for human health and food safety respond to these events through the development of enhanced enteric illness surveillance networks, including the use of molecular subtyping and other laboratory technology, to enable cluster detection and the linkage of seemingly unrelated cases to initiate outbreak investigation. Ongoing public awareness of food safety demands the swift resolution of food safety issues at a time when they are becoming increasingly complex, reinforcing the need for collaboration in multi-jurisdictional outbreak investigations and the active participation of all partners in centrally led efforts to mitigate risk and prevent further illness.
A structured approach to managing multi-jurisdictional foodborne illness outbreaks helps to streamline roles and actions, thereby protecting the health of Canadians. The FIORP is the principal framework document that guides multi-jurisdictional collaboration in response to foodborne illness outbreaks in Canada.
3. Purpose
The FIORP is intended to be used to coordinate the actions of multiple agencies in response to foodborne illness outbreaks that span more than one P/T or involves Canada and another country.
The purpose of the FIORP is to set out the key guiding principles and operating procedures for the identification and response to multi-jurisdictional foodborne illness outbreaks in order to enhance collaboration and coordination among partners, establish clear lines of communication, and improve the efficiency and effectiveness of response. It is not intended to provide detailed instructions on how to conduct investigation and response.4. Scope
The FIORP describes activities beginning with the notification and assessment of a potential multi-jurisdictional foodborne illness outbreak and ends with either the containment of the risk that triggered the outbreak or resolution of the outbreak.
The FIORP is intended to be used for foodborne illness outbreaks that affect, or have the potential to affect, more than one P/T or affect Canada and another country or countries. It is complementary to agreements and procedures established within individual agencies with roles in foodborne illness response, including P/T foodborne illness outbreak response protocols. Where more than one country is affected, the FIORP is intended to guide activities within Canada only.
The FIORP addresses potential foodborne illness outbreaks resulting from the natural, accidental, or intentional contamination of foods by biological, chemical, or physical substances.
The principles outlined in the FIORP also serve as a guide when human enteric illness outbreaks are caused by contact with animals or pet food (e.g. contaminated pet food and treats, petting zoo animals, contact with pets such as reptiles, rodents, backyard poultry, etc.) or when other food hazards cause widespread human injuries requiring prompt collaboration and coordination (e.g. inert physical hazards). Further guidance on the response to multi-jurisdictional enteric illness outbreaks linked to contact with animals or pet foods can be found in Annex 14.
The FIORP does not specifically address the broader risk assessment process that contributes to policy development and standard-setting to reduce the risk of future outbreaks, however the opportunity to raise the need for future policy development is provided for during the post-outbreak debrief/review.
5. Guiding principals
- Protecting the health of Canadians
The primary objective of the activities described in the FIORP is to mitigate or contain the effects of a foodborne illness outbreak in a timely and effective manner, thereby protecting the health of Canadians. - Sharing information in a timely manner
Subject to applicable laws governing the sharing of information (including privacy, access to information and common law relating to confidential business information), the partners recognize that information required to investigate, control, and resolve a foodborne illness outbreak will be exchanged in confidence and in a timely fashion between the partners. - Public disclosure of information
The partners recognize that public disclosure of confidential business information may be required when a foodborne illness outbreak, or foodborne health hazard that could pose a risk to public health, is identified and there is a clear public interest in sharing this information. The response to external requests for information should be coordinated between affected OICC partners and align with applicable access to information and privacy legislation. - Using the Outbreak Investigation Coordinating Committee (OICC) as the central body for coordination and information sharing
The OICC established pursuant to this FIORP will serve as the main forum for information sharing and interpretation, clarification of roles and responsibilities, establishment of response priorities, and the development of communications strategies related to an actual or suspected foodborne illness outbreak. While some discussions may need to occur outside of the OICC, all activities, recommendations and decisions will feed back to the OICC in a transparent and timely fashion. - Providing assistance to partners
Whenever possible, the partners implementing the FIORP will provide assistance, including laboratory support, as requested during an epidemiological investigation or food safety investigation. - Respecting other agreements and relationships in place
The FIORP is intended to complement agreements and procedures established among the partners. Where memoranda of understanding (MOU) or agreements between the partners, regarding food safety surveillance, investigation or control, may exist or are negotiated, these will be shared and respected. The FIORP is not intended to substitute for the ongoing relationships between the partners necessary to discharge other responsibilities and to manage issues as they arise. - Weight of evidence
Laboratory, epidemiological, or food safety investigation evidence is accepted for establishing the association between a particular food or foods and human illness. - Active engagement in FIORP
The partners are encouraged to raise awareness of the FIORP within their own jurisdiction by distributing the document to their senior management and foodborne illness outbreak response partners and by participating in OICCs as appropriate, and simulation exercises/training where possible. - International Health Regulations
Canada, including the P/Ts, is responsible for ensuring that its obligations pursuant to the International Health Regulations (IHR 2005) are met. - Publication
Publication of information related to multi-jurisdictional foodborne outbreaks investigated collaboratively through an OICC will not occur without the permission of all the partners engaged in the investigation and response whose data will be included in the publication.
6. Roles and responsibilities
Responsibilities for responding to foodborne illness outbreaks are shared between F/P/T and local/regional jurisdictions. The response to such situations involves collaboration and cooperation among all those involved. Annex 12 describes the legislative authorities within each of the F/P/T governments and provides more detailed roles and responsibilities of all the partners.
6.1 Federal authorities
Under the federal Minister of Health, PHAC, HC, and the CFIA have legislated responsibilities for responding to foodborne illness-related events.
6.1.1 Public Health Agency of Canada
PHAC coordinates the multi-jurisdictional outbreak response in collaboration with affected partners, conducts national laboratory-based surveillance, provides expertise to public health officials, provides advice to Canadians during an outbreak, and builds capacity for responding to enteric illness outbreaks. PHAC also acts as the International Health Regulations (IHR) national focal point, which is the national centre designated to communicate with the World Health Organization (WHO) IHR Contact Points under the regulations.
Within the Government of Canada, the CFEZID Outbreak Management Division (OMD) at PHAC is the usual first point of contact for notification by the partners of issues related to actual or potential foodborne illness outbreaks and requests for content expertise/support for foodborne outbreak investigation. In international foodborne illness outbreak situations, CFEZID will act as the main liaison with international public health counterparts. The Centre plays the following role:
- The Enteric Surveillance and Population Studies division (ESPS) conducts national surveillance for enteric illnesses and collaborates with international surveillance activities;
- OMD coordinates multi-jurisdictional foodborne illness outbreaks involving more than one P/T or involving Canada and another country or countries where appropriate;
- OMD provides consultation and content expertise in other foodborne outbreak investigations as requested;
- OMD interprets and comments on the weight of epidemiologic evidence collected during the investigation of enteric illness outbreaks with a food source;
- OMD and ESPS provide training in enteric outbreak investigation methods.
The National Microbiology Laboratory (NML) provides reference services for strain identification and characterization, national laboratory-based surveillance, and dissemination of information through PulseNet Canada and the National Enteric Surveillance Program (described in Annex 8). The NML, through PulseNet Canada, is the usual first point of contact for P/Ts sharing strain identification data and the detection of clusters of strains that are occurring in more than one P/T, indicating the potential for multi-jurisdictional foodborne outbreaks.
The Canadian Field Epidemiology Program and the Canadian Public Health Service is also available to provide additional epidemiology surge capacity resources that can be mobilized to assist in the investigation of enteric illness outbreaks.
The Travelling Public Program (TPP), as part of its risk-based public health inspections on passenger conveyances (e.g. aircraft, trains, ferries, and cruise ships) and their ancillary services (e.g. flight kitchens), administers and enforces food safety provisions (sections 4 and 7) of the Food and Drugs Act on behalf of the CFIA. TPP also provides environmental quarantine services, and conducts ship sanitation inspections pursuant to the IHR.
6.1.2 Health Canada
HC is the federal department responsible for setting the regulations and standards for the safety and nutritional quality of food sold in Canada. Its food safety responsibilities include:
- establishing policies, regulations and standards related to the safety and nutritional quality of all food sold in Canada – Food Directorate;
- regulating pesticides – Pest Management Regulatory Agency;
- managing human health and safety risks associated with consumer products – Consumer Product Safety Directorate;
- evaluating the safety of veterinary drugs used in food-producing animals - Veterinary Drugs Directorate; and
- Food safety in First Nations communities south of 60 degrees parallel – First Nations Inuit Health Branch (FNIHB).
HC may be involved or assist with investigations of foodborne illness outbreaks as follows:
The Food Directorate focuses on issues relating to microbial pathogens, chemical contaminants, marine biotoxins, undeclared food allergens or other potential health hazards in foods. Specifically, the Food Directorate provides:
- health risk assessments (HRA) on food-related hazards to the CFIA or other stakeholders (e.g., P/T governments)
- scientific advice and analytical surge capacity for analyzing microbiological contaminants, chemical contaminants, non-permitted food additives, chemicals associated with the use of food packaging materials, processing aids, and incidental additives, and undeclared food allergens in food and clinical samples;
- national reference services for foodborne botulism, listeriosis, as well as Vibrio, viruses and parasites; and
- risk management advice, including public communication.
The Pest Management Regulatory Agency provides, upon request, HRAs on pesticide residues exceeding the legal limits to the CFIA or other stakeholders. It also contributes to investigations involving incidences of pesticide residues above the legal limits.
The Consumer Product Safety Directorate, under the Canada Consumer Product Safety Act, helps address and prevent dangers to human health and safety that are posed by consumer products in Canada.
The Veterinary Drugs Directorate is responsible for setting maximum residue limits for veterinary drugs in foods.
The FNIHB provides support and technical advice in the investigation of foodborne illnesses for First Nations communities on reserves south of 60 degrees parallel. The Environmental Public Health Division within FNIHB is the national contact point between the FNIHB regional offices and other involved parties (e.g., the CFIA) during a suspected or confirmed foodborne outbreak in First Nations communities. FNIHB regional staff disseminate food recall information issued by the CFIA, carry out food safety investigations in food establishments, conduct visits at facilities with vulnerable populations (e.g., daycare, treatment centres, hospitals), and provide public education and food handler training sessions, as needed, in affected First Nations communities.
6.1.3 Canadian Food Inspection Agency
The CFIA delivers all federal inspection and enforcement services related to food under the authority of 13 federal acts that address all stages of the food continuum. Not only does the CFIA inspect foods, but also the seed, livestock feed, fertilizers, plants, and animals on which a safe food supply depends. The CFIA contributes to the investigation and control of foodborne illness outbreaks by conducting food safety investigations, testing and recall activities, as well as its regulatory compliance and enforcement activities. The CFIA acts as the main point of contact with international food safety authorities when a foodborne illness outbreak involves Canada and another country.
The CFIA’s role in food safety investigations includes tracing foods from the retail level through distribution to production or processing facilities to pinpoint a suspected source of the problem. Information obtained throughout the food safety investigation provides the basis for the assessment of risk and the development of appropriate risk management strategies to control affected products. The food industry carries out most recalls voluntarily. However, if a company is not available or willing to conduct the recall voluntarily, the Minister of Health can, under the Canadian Food Inspection Agency Act, order a company to recall a product where the Minister believes that it poses a risk to public, animal, or plant health. In the case of voluntary recalls, the CFIA officials will verify that the recalling firm has recalled the product effectively.
When a potentially contaminated food that could pose a risk to the public has been identified in Canada, the CFIA launches a food safety investigation to:
- determine the nature, extent and cause of the problem;
- confirm whether a health hazard exists; and
- identify the appropriate risk management options.
This work is done collaboratively with P/T partners and is guided by MOUs.
There are three groups within the CFIA that play key roles in the food safety response to foodborne illness outbreak situations:
- Regional inspection staff, including Area/Regional Recall Coordinators (ARCs/RRCs), are involved in food safety inspection activities. The ARCs/RRCs are also the usual first point of contact within the CFIA for local/regional health units and P/Ts.
- The Office of Food Safety and Recall (OFSR) is responsible for the coordination and consistency of decision-making on food safety issues and recalls, and provides the link with HC for obtaining HRAs assessments as appropriate. The OFSR is the usual first point of contact for national and international food safety related issues.
- The Laboratory Coordination Division of the Food Safety Science Directorate is responsible for providing scientific guidance to CFIA staff and P/T partners by coordinating food sample delivery within the CFIA’s laboratory network and providing interpretation of laboratory analyses and results.
6.2 Provincial/ Territorial and Local authorities
Local/regional health officials generally have the mandate to investigate and control human illness outbreaks that occur within their boundaries, with local/regional medical officers of health (where applicable) taking a leadership role. In some jurisdictions, other departments (e.g. Agriculture) may also have a role in foodborne illness investigations. Additionally, local/regional health officials have the responsibility to report enteric illnesses to P/T health officials under disease control legislation.
P/T officials conduct enteric illness surveillance, support local/regional health officials in investigating and controlling outbreaks, and may also carry out inspection and education activities to reduce the risk of enteric illnesses. Some P/Ts have their own foodborne outbreak response protocols to guide the collaborative response within the P/T and identify the lead should an outbreak span local/regional boundaries. In some P/Ts, food regulatory officials also participate in or lead the investigation. In addition, the territories have responsibilities for the investigation of enteric illness outbreaks that occur in First Nations communities north of 60 degrees parallel.
Local/regional or P/T officials may also, in some cases, request the assistance of HC, PHAC, or the CFIA in the response to a potential enteric illness outbreak.
The P/Ts provide the case-level information required for the centralized collation and analysis of data by the OICC lead agency in order to inform decision-making and draw conclusions based on all available evidence during a multi-jurisdictional foodborne illness outbreak.
6.2.1 Single jurisdiction outbreaks
The FIORP is intended to be used for foodborne illness outbreaks that span more than one P/T or involves Canada and another country. Outbreaks occurring in a single jurisdiction are managed by local/regional or P/T officials as per the established protocols or agreements for the respective jurisdiction. Public communications about human illnesses and recommended public health measures will be led by the implicated jurisdiction; the government authority handling the recall or other control measures (affected P/T or CFIA) will lead on food recall communications.
Federal authorities may become involved in single jurisdiction foodborne illness outbreaks in various ways. The most common examples are:
- Requests for assistance (e.g. resource support, technical expertise)
- Food safety inspection activities via CFIA regional inspection staff
- Request to HC for a health risk assessment (Section 7.7)
While many single jurisdiction outbreaks will not evolve into multi-jurisdictional outbreaks, officials should consider the factors listed in Section 7.2 in deciding whether to notify federal partners of the single jurisdiction outbreak. This initial notification will lead to a review of the available information to determine if a multi-jurisdictional foodborne outbreak exists (Section 7.3.1).
6.3 Other agencies and organizations
Expertise from other F/P/T or international agencies may be sought to provide advice in the control of outbreaks caused by unusual pathogens or toxic substances in foods. Some agencies (e.g., Correctional Service Canada, National Defence and the Canadian Armed Forces) may be actively involved if the illnesses are affecting federal populations. Key international partners can include the WHO, Pan American Health Organization, U.S. Centers for Disease Control and Prevention, and other public health and regulatory agencies as appropriate.
If an outbreak is suspected to be related to criminal activity (e.g., tampering and terrorism), law enforcement agencies (local police or the Royal Canadian Mounted Police (RCMP)) assume responsibility for the law enforcement response and the criminal investigation (Section 7.8.1).
7. Operating procedures
The following sections outline the general operating procedures for coordinating the response to a potential multi-jurisdictional foodborne illness outbreak. Figure 1 provides a schematic overview of how the FIORP operates.
Figure 1 – How the FIORP operates
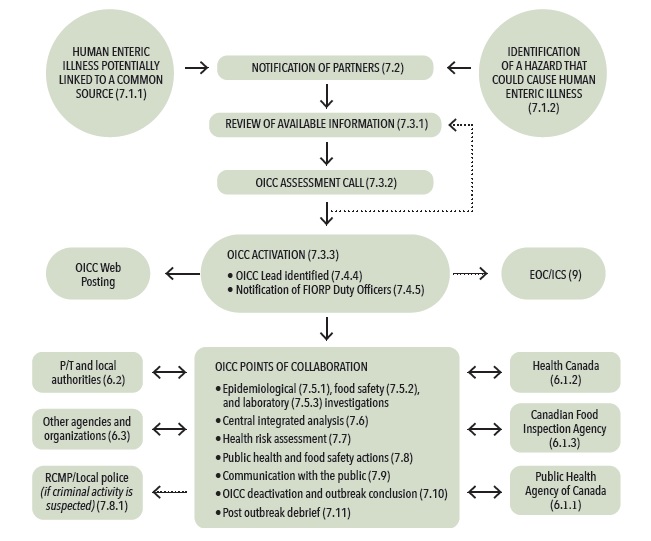
Figure 1: How the FIORP operates.
Figure 1 represents the general operating procedures for coordinating the response to a potential multi-jurisdictional food-borne illness outbreak.
The procedure would typically begin with the notification of partners of an issue with the potential to become a multi-jurisdictional food-borne illness outbreak.
A potential multi-jurisdictional food-borne illness outbreak may come to the attention of public health or food regulatory agencies through notifications from partners, reports of human illness (surveillance), a routine inspection that reveals a potential for human illness, or a food safety investigation.
Once a potential multi-jurisdictional food-borne illness outbreak has been identified, there is a requirement to examine the current available information and determine if it is sufficient to indicate the presence of a potential multi-jurisdictional food-borne illness outbreak that requires a collaborative investigation and the activation of an Outbreak Investigation Coordinating Committee (OICC).
An OICC Assessment call will be held among affected partners (those with cases of human illness or relevant food-borne hazard information) to review the available information and assess whether or not an OICC is required.
If the partners agree that an OICC is not required at that time, partners will continue to monitor the cluster. An OICC assessment call can be held if new information warranting collaborative assessment becomes available.
If the initial assessment and review of available information indicates that an OICC should be activated, the OICC lead will initiate a teleconference call with the affected partners' identified representatives to activate the OICC and begin the coordination of the investigation. The FIORP duty officers will be notified by the OICC lead and asked to inform their senior officials. In addition, PHAC will pro-actively add the investigation event to foodborne illness outbreaks webpage on Canada.ca.
Most multi-jurisdictional foodborne illness outbreak investigations do not require the use of an incident command system (ICS) and activation of emergency operations centres (EOC). However, agencies may consider using such an approach to help coordinate the response if a foodborne outbreak becomes a public health emergency.
The composition of and collaboration with an OICC involves stakeholders such as provincial/territorial officials, local/regional officials, HC, the CFIA, PHAC, law enforcement (if required), and other agencies and organizations, as needed. The composition of the OICC will depend on the nature of the outbreak and may evolve as knowledge related to the source of the outbreak is generated during the outbreak. At varying times, it should have representatives that provide epidemiological, food safety, laboratory, and communication expertise from the different levels of government required.
The OICC's points of collaboration includes
- coordinated investigations, including epidemiological, food safety, and laboratory investigations;
- central integrated analysis;
- health risk assessment;
- public health and food safety actions;
- communication with the public;
- OICC deactivation and outbreak conclusion;
- post outbreak debrief.
7.1 Identification of a potential multi-jurisdictional foodborne illness outbreak
A potential multi-jurisdictional foodborne illness outbreak may be identified through reports of human illness (surveillance) or the identification of a hazard that could cause human enteric illness. Examination of surveillance data and the determination of cases in more than one jurisdiction could prompt further investigation and notification of affected partners.
7.1.1 Human Enteric Illness potentially linked to a common source
Human health surveillance activities occur at the local/regional, F/P/T, and international levels. Increased or unusual cases of human illness may trigger investigations to determine a common source. Identification of human enteric illnesses potentially linked to a common source may originate from the following sources:
- Outbreaks recognized by local/regional officials through increased reporting of a particular enteric pathogen or complaints of enteric illness linked to a common exposure;
- Routine enteric illness surveillance activities at the national or P/T level indicating that a P/T or national enteric outbreak is in progress; or
- International enteric illness outbreaks identified through PHAC’s network activities with international groups (e.g. the U.S. Center for Disease Control and Prevention, PulseNet International, the WHO, the media, notification from foreign public health or food safety authorities).
7.1.2 Identification of a hazard that could cause Human Enteric Illness
Food safety investigations may be triggered by the following situations:
- Routine sampling and testing activities that detect the presence of a hazardous contaminant (biological or chemical) in a distributed food;
- Consumer complaints concerning a food, which may involve reports of illness;
- Deviations in food preparation, processing, storage, and transport identified during inspection activities;
- Notification from industry (manufacturer, processor, distributor, importer, common carrier, etc.) of a potential food safety problem; or
- Information about a food safety problem from other external sources (e.g. foreign health officials, industry or public health associations, academia).
7.2 Notification of partners
Notification refers to the initial contact between partners to identify an issue with the potential to become a multi-jurisdictional foodborne illness outbreak. Notification can occur through different means and involves the exchange of public health and food safety information. One mechanism of notification is Public Health Alerts, an effective communication tool on the Canadian Network for Public Health Intelligence (CNPHI) portal used for early notification of potential outbreaks (Annex 2). A FIORP contact list of federal and P/T partners is maintained by PHAC and updated on a quarterly basis. To request a copy of the list, please contact CFEZID at PHAC by e-mail: fiorp.mitioa@phac-aspc.gc.ca.
Officials at any level of government (local, P/T, or federal) should consider the following factors in deciding whether to notify affected partners:
- Illnesses are, or have the potential to be, spread over more than one geographic jurisdiction (multiple P/Ts, or within Canada and another country or countries);
- An unusual or particularly pathogenic organism is suspected/involved;
- The outbreak is known to be, or has the potential to be, related to a widely distributed food item;
- A significant number of unexplained illnesses are involved;
- Intentional contamination is suspected;
- The outbreak may constitute a public health emergency of international concern as described in the IHR (2005).
If notification of international partners is required, the responsible federal partner will act as a liaison with foreign countries. In international foodborne illness outbreak situations, PHAC (CFEZID) will act as the main liaison with international public health counterparts. The CFIA will act as the main liaison with international food safety counterparts for international food safety related issues.
7.3 OICC assessment call and OICC activation
7.3.1 Review of available information
Once a potential multi-jurisdictional foodborne illness outbreak has come to the attention of public health or food regulatory agencies, there is a requirement to examine the current available information and determine if a multi-jurisdictional foodborne outbreak exists. Each affected partner should make efforts to gather, summarize, and share the information available to them prior to an OICC assessment call. A suggested template for information that could be shared on an OICC call is provided in Annex 1
7.3.2 OICC assessment call
A teleconference call will be held among affected partners (those with cases of human illness or having relevant foodborne hazard information) to review the available information and decide whether an OICC should be activated. If the partners agree that an OICC is not required at that time, further OICC assessment calls can be held if new information warranting collaborative assessment becomes available.
An assessment call typically includes representatives from the following partners: PHAC’s CFEZID Outbreak Management Division (chair of teleconference); PHAC NML; HC Bureau of Microbial Hazards; CFIA-OFSR; and public health/epidemiology and laboratory representatives from P/Ts with cases. Additional partners may be included as required and include federal contacts in specific program areas, P/T agricultural agencies and local public health authorities.
7.3.3 OICC activation
The following considerations are made when deciding whether an OICC should be activated:
- Cases are occurring in multiple P/Ts or occurring in Canada and another country or countries
- The outbreak is known to be, or has the potential to be, linked to a common source
- The outbreak requires or will benefit from the use of the FIORP to enhance collaboration, sharing of information and coordinating actions and communications.
Consideration may also be given to the severity and scope of the potential multi-jurisdictional foodborne illness outbreak, such as:
- a larger than expected number of cases linked by laboratory evidence;
- new cases continue to be identified;
- severe illness or deaths observed among identified cases;
- an unusual or particularly pathogenic organism is suspected/involved; and/or
- a vulnerable population is over-represented among cases (e.g. all children).
Any partner involved in a foodborne illness or food safety investigation with potential multi-jurisdictional outbreak implications can request that the OICC be established under the leadership described in Section 7.4.4. The decision to activate an OICC is based on consensus where possible. Where consensus cannot be achieved, the OICC activation will proceed if the majority of partners agree to OICC activation and there are no strong objections raised by dissenting partners. In instances where there are strong objections that cannot be resolved, guidance from senior officials can be sought.
An identified foodborne hazard in the absence of human illness or widespread injury would not trigger OICC activation. When a potentially contaminated food has been identified in Canada that could pose a risk to the public, the CFIA will launch a food safety investigation (Section 7.5.2).
When an OICC is activated, a notification is sent to the FIORP Duty Officers, CCMOH, and P/T epidemiology and laboratory representatives, and PHAC will pro-actively add the investigation event to foodborne illness outbreaks webpage on Canada.ca. Further detail on how the OICC operates is provided in the following section.
7.4 Outbreak Investigation Coordinating Committee (OICC)
A central element of the FIORP is the establishment of an OICC, with representation from the partners who are actively involved in a specific outbreak, to coordinate a multi-agency response to a foodborne illness outbreak.
7.4.1 Purpose of the OICC
The OICC’s primary objectives are to:
- facilitate communications among participating organizations;
- clarify roles and responsibilities of partners specific to the incident at hand;
- serve as a central point to share information from all sources and discuss findings, including results of centralized data analysis;
- make decisions on investigative approaches;
- communicate outbreak response strategies and coordinate investigations among the partners, such as follow-up and corrective actions;
- identify resource needs and opportunities for sharing resources;
- establish priorities for response where critical resources are limited or constrained;
- gain consensus in resolving issues that emerge; and
- develop comprehensive external communications strategies, ensuring the release of consistent and complementary messages to the public and other stakeholders (see Annex 10).
7.4.2 Composition of the OICC
An OICC will be comprised of representatives designated to act on behalf of the partners involved in the foodborne illness outbreak investigation. Representatives should have the authority to make decisions related to technical and operational issues and have access to senior decision-makers for issues with policy implications. It is the responsibility of each of the partners to determine its own appropriate representation on the OICC. The partners should strive to limit representation on the OICC to the responsible parties required for investigation and response to the outbreak.
The composition of the OICC will depend on the nature of the outbreak, and it may evolve as knowledge related to the source of the outbreak is generated during the outbreak. It should have representatives that provide epidemiological, food safety, laboratory, and communication expertise from the different levels of government required.
Partners may include the following:
- PHAC;
- CFIA:
- HC;
- P/T partners;
- Local public health units; and
- Other agencies, as required.
7.4.3 Decision-making and resolving differences of opinion
The OICC will strive to make consensus-based decisions on strategies for response, while recognizing that each partner has unique legal obligations, policies, and mandates that must be respected. Any decisions made by one of the partners pursuant to its obligations, but related to the purpose of the OICC, should be communicated to all OICC partners. All feedback put forward by OICC partners will be taken into consideration in arriving at a final decision.
The OICC will attempt to resolve all differences of opinion during the course of an outbreak. However, when consensus cannot be reached or when further risk management guidance is needed, the partners should seek guidance from senior officials in their respective agencies through their identified FIORP Duty Officer. Senior officials should confer together if possible. The OICC will determine whether engagement of formal senior decision-making bodies (e.g. CCMOH, others) is warranted; senior public health officials may also choose to convene if they feel it is necessary (Section 7.4.6). Any decision made by senior officials in resolving the issue should be communicated to all OICC partners.
7.4.4 The OICC lead
Once the OICC is activated, the lead organization responsible for coordinating an OICC (OICC lead) will be identified using these guidelines:
- If an outbreak involves more than one P/T or has an international dimension (occurs in Canada and another country or countries), PHAC (CFEZID) will be considered the OICC lead.
- PHAC (CFEZID) may defer the OICC lead to a P/T upon agreement by all OICC representatives should an outbreak occur primarily within that P/T and a formal outbreak investigation team has already been established. The OICC lead responsibilities to be retained by PHAC in this scenario would be negotiated with the P/T OICC lead
- P/T representatives on the OICC may continue to lead the internal P/T response within their respective jurisdictions
- The transfer of leadership from the P/T(s) to PHAC, if applicable, will occur once the OICC is activated.
Any partner may request that an OICC be coordinated under the leadership described above.
Responsibilities of the OICC lead include:
- notifying the FIORP duty officers (Section 7.4.5) of the activation and deactivation of an OICC and providing updates after OICC calls
- centrally collating and analyzing data (Section 7.6)
- managing meetings (Annex 1), including identification of a chair for the OICC teleconference calls
- recording and distributing discussion summaries and action items to the OICC partners and FIORP duty officers
- maintaining documentation of the response effort
- deactivating the OICC and declaring the outbreak over (if applicable)
- organizing the post-outbreak debrief, if required.
7.4.5 FIORP duty officers
Each partner will identify a named position within its organization to serve as a FIORP duty officer. All FIORP duty officers will be notified by the OICC lead when an OICC is activated and deactivated, and will receive updates after OICC calls. FIORP duty officers are responsible for ensuring that senior officials within their organization are appropriately briefed and that their organization leads or participates in an OICC as required. If the FIORP duty officer is not a participant in the OICC, discussions with their organization’s OICC representative should occur to clarify briefing responsibilities. Contact information for the FIORP duty officers will be maintained by PHAC as part of the FIORP contact list.
7.4.6 Engagement of Senior Public Health Officials
In some situations (e.g., exceptional outbreaks involving serious human health implications or garnering significant public, media or political interest), the Chief Public Health Officer of Canada and one or more CMOHs may choose to convene outside of the OICC to discuss aspects of outbreak management. These aspects may include, but are not limited to, addressing specific issues related to the public health actions and public communications. When a significant multi-jurisdictional foodborne illness outbreak is identified, a meeting of the CCMOH can also be considered.
- A member of the CCMOH can request, through a decision from the Chair, that the CCMOH be convened at any time during a significant multi-jurisdictional foodborne illness outbreak.
- A technical representative from the OICC lead agency will participate in the CCMOH meetings to ensure continuous coordination and communication with the OICC. This OICC representative will report back to the OICC on CCMOH key actions and decisions.
- The CCMOH Secretariat will support CCMOH meetings including documenting key action items and decisions for distribution to CCMOH members and the OICC lead.
7.5 Coordinated investigations
7.5.1 Epidemiological investigations
To facilitate epidemiological investigations of multi-jurisdictional foodborne illness outbreaks, the OICC will assess what case-level information is required and determine which partner is best able to gather the identified information. Every effort will be made to standardize the collected information. Data analysis will occur within each jurisdiction and agency as per standard protocols. However, when multiple partners are involved, the overall collation and analysis of epidemiological data will take place within the identified OICC lead. The OICC will discuss what type of analysis will best support the examination of findings from all aspects of the outbreak investigation.
7.5.2 Food safety investigations
When the source of an outbreak is suspected to be a food, a food safety investigation will be conducted to determine whether the food may be responsible for the outbreak and to strive to identify the root cause of the contamination in the affected food.
If the food is imported or shipped interprovincially or manufactured in an establishment under the CFIA's jurisdiction, the CFIA will conduct the food safety investigation.
If the food is produced or manufactured in a facility that received a licence or registration from a P/T or regional/local authority, or where the CFIA has signed an MOU with a P/T concerning shared responsibilities for inspection, the partner who has jurisdiction may conduct the food safety investigation or it may be conducted jointly with the CFIA. Assistance may be requested from other regulatory partners.
Should the food safety investigation expand to include issues of employee health, where employee records of illness and/or employee test results are required, the responsible regulatory officials should request the assistance of the appropriate public health authority in the jurisdiction where the investigated facility is located.
7.5.3 Laboratory investigations
Both epidemiological and food safety investigations usually involve laboratory testing. Each of the partners is responsible for conducting the appropriate laboratory analyses as part of its respective investigation and mandate. The OICC coordinates laboratory analyses in order to identify the most appropriate tests to be done, avoid overlap and duplication, permit discussion of issues, and share results.
In some cases a partner may not have the necessary capacity or expertise to perform the necessary test(s). It should then contact supporting laboratories (refer to Annex 9 for detailed guidance on laboratory capability and instructions for access) in order to send the samples to a laboratory that has the required expertise and capacity. The process for directing food samples to the federal laboratory network is outlined in Annex 13.
The use of PulseNet or other existing laboratory networks should facilitate communication among F/P/T laboratories.
| Other suspected agents | Where to send samples if testing capability is not available within P/T |
|---|---|
| Clostridium botulinum | Botulism Reference Service (Annex 4) |
| Listeria monocytogenes | Listeria Reference Centre for Canada (Annex 5) |
| Food-related viruses | Food Virology Reference Centre for Canada (Annex 6) |
7.6 Centralized integrated analysis
When multiple partners are involved, centralized collation and analysis of data by the OICC lead is required to inform decision-making and draw conclusions based on all available data. Findings from the epidemiological, laboratory, and food safety investigations will be shared by and with the OICC partners and integrated by the OICC lead to identify the potential cause and source of the outbreak and areas for further investigation.
7.7 Health risk assessment
HC is mandated to provide HRAs on microbiological hazards associated with food safety investigations/incidents. HRAs may be requested by CFIA and/or by the P/Ts and other public health or food safety authorities during a coordinated outbreak investigation to inform risk management activities.
In foodborne illness outbreaks, HC uses the approach described in the “Weight of Evidence: Factors to Consider for Appropriate and Timely Action in Foodborne Illness Outbreak Investigations”. Information arising from the various coordinated investigations, outlined in Section 7.5, is used to further inform the streams of evidence assessed in the weight of evidence approach. The evidence gathered is analyzed and a weight given to the various factors that contribute to each of the three streams of evidence, i.e., epidemiological evidence (Annex 3), food safety investigation and microbiological evidence. The weight of evidence approach is then used by HC to determine if a level of health risk can be assigned to a food and initiate the HRA process, if appropriate. Roles/responsibilities for gathering evidence and preparing HRA documentation in OICC-coordinated investigations are established by the OICC.
The HC HRA process follows the guidelines developed by the FAO/WHO Codex Alimentarius Commission 14, which is responsible for developing international food standards and guidelines. Decisions and rationales are conveyed to the requesting authorities and the outputs are also shared with the OICC to facilitate its coordination role. HC participation facilitates the exchange of information and provision of scientific advice to support the HRA process.
7.8 Public health and food safety actions
Actions undertaken during a foodborne illness outbreak to address the source of the outbreak and prevent further cases of human illness may include a wide range of activities by one or more of the partners. Examples include:
- recalling, detaining, or disposing of a contaminated food product;
- public communication outlining recommended prevention and control activities;
- inspection, closure, sanitation, and review of practices at implicated facilities;
- case and contact management; and
- provision of prophylaxis (e.g., vaccination for Hepatitis A contacts).
Each partner will conduct the necessary mitigation actions under its respective mandate. The OICC coordinates information sharing related to these actions and facilitates discussions concerning the timing of these actions.
7.8.1 Tampering
In the event that a multi-jurisdictional foodborne illness outbreak investigation identifies or suspects the intentional contamination of a food product, the appropriate local/regional law enforcement agency must be immediately notified. Regardless of police jurisdiction, the RCMP National Operations Centre must also be contacted at 613-993-4460.
Following notification of the appropriate authorities, the OICC would continue to coordinate the outbreak investigation in collaboration with law enforcement authorities, who may conduct a criminal investigation.
7.8.2 Exchange of industry information
a) Exchange of information with industry
During an investigation, all implicated companies will be kept informed of developments by the responsible inspection authority.
The CFIA is the responsible inspection authority and primary contact, with processors and importers operating under federal jurisdiction unless there is a signed MOU assigning that role to a P/T. However, for processors operating under P/T jurisdiction or where the CFIA has signed an MOU with a P/T concerning shared responsibilities for inspections, the appropriate P/T officials would be the primary industry contact unless otherwise agreed.
Some outbreaks may require communication with industry representatives beyond the implicated facility. In this case, the OICC will identify the appropriate partner to be the lead communicator to these industry representatives, according to the partners' mandates and jurisdictions. The lead communicator should consult with the OICC about what outbreak investigation information should be shared with industry representatives beyond the implicated facility, and the rationale for sharing the information.
b) Exchange of industry information between OICC partners
The responsible inspection authority will share relevant information stemming from its investigation with other investigating organizations through the OICC, as appropriate. The exchange of information among government agencies will be conducted according to Guiding Principle II of the FIORP.
7.9 Communication with the public
7.9.1 Key Principles of public communications
In the event of a foodborne illness outbreak, the principles of risk communications will be used to guide public communications messaging and activities. The key principles include:
- Communicating as a priority where there is the opportunity to protect health by providing the public with information that will help them protect themselves and/or others;
- Considering citizen and stakeholder information needs, preferences, and requirements as part of the decision-making process;
- Being open, transparent, empathetic, and timely, unless there is a valid reason to withhold information (i.e. drastic change is expected in the next 24 hours, violation of privacy laws or confidentiality agreements, legal risks, etc.);
- Where possible, basing communications strategies and tactics on natural and social science; and
- Building public trust in the capacity of the organization by sharing information and messaging that will clarify a situation, acknowledge uncertainties, provide advice, and explain what may happen next.
7.9.2 Responsibilities and leads
Each of the partners has the responsibility to communicate with the general public within its respective jurisdiction and to designate a spokesperson when an outbreak investigation has been initiated. The objective is to coordinate, where appropriate, public communications to ensure consistency of messaging (thereby building public trust) and to broaden the message reach.
The organizational lead for public communications will depend on the situation involving the foodborne illness outbreak. If the outbreak is occurring within one province or territory, the implicated jurisdiction will lead on public communication on human illnesses associated with the outbreak, and recommended public health measures. In this situation (single jurisdiction event) the government authority handling the recall or other control measures (affected P/T or CFIA) will lead on food recall communications.
In a multi-jurisdictional outbreak, an international event, or on a conveyance inspected by TPP, public communications related to human illnesses and public health measures will be led by PHAC; food recall communications will be led by the CFIA; and public education related to safe food handling will be led by HC.
Due to the nature of foodborne illness outbreaks, all involved partners have a responsibility to coordinate communications activities in a consistent and timely way. In instances when communications coordination is required, an Outbreak Communications Team (OCT) is established to guide this process and to ensure all partners are engaged and made aware of all public communications activities being undertaken by any of the OICC partners (see section 7.9.4 Outbreak Communications Team).
Communication to health professionals may also be required as part of the response to a multi-jurisdictional foodborne outbreak. This communication will be coordinated as part of the OICC investigation and response activities. Distribution of the communication products to health care professionals remains the responsibility of the P/Ts.
7.9.3 Coordination among involved F/P/T partners
When a multi-jurisdictional OICC has been established, a foodborne illness outbreak investigation web posting will occur on Canada.ca. Communications representatives from all OICC partners involved in the outbreak event will be integrated into the OICC to provide advice and share information about further communication activities related to the outbreak. At the first OICC teleconference, each F/P/T partner involved in the foodborne illness investigation will appoint a communications lead within their organization to act as a member of the OCT for the duration of the event. The OCT will be led by the communications representative from the organization leading the foodborne illness outbreak investigation (see 7.9.2 Responsibilities and Leads.)
In the event that F/P/T EOCs are activated, the appropriate communications representatives from the OCT will be integrated into those structures to maintain timely information-sharing.
Each lead organization involved in the OICC will identify a designated spokesperson. OICC partner organizations may communicate with the general public within their respective jurisdictions.
7.9.4 Outbreak communications team
The objective of the OCT is to coordinate a public communications approach among all OICC partners involved in a foodborne illness outbreak event. When the need for public communications has been discussed and a communications approach is determined within the OICC, members of the OCT will be notified by the OCT lead by email regarding the OICC’s proposed approach and be asked to engage with their organization’s OICC representatives regarding input and feedback on draft communications products.
For outbreaks requiring further discussion about the communications approach beyond an OICC teleconference, a separate communications teleconference will be convened by the OCT lead, including all OCT members and the OICC lead. This group will be brought together to discuss communication tactics, key messages, and timing to ensure a coordinated approach is taken by all members. The OCT lead (as outlined in section 7.9.3 Coordination Among Involved F/P/T Partners) will be responsible for developing coordinated plans, writing products and drafting messaging for communicating with the public and those at greater risk. All OCT members will be responsible for:
- sharing information with their respective OICC representatives and the OCT lead – including details from their jurisdiction/organization that could impact the communications approach, products, media relations, or other jurisdictions– on an ongoing basis during an outbreak;
- providing communications advice and support to their organization’s OICC representatives regarding the communications approach for an outbreak;
- communicating to the OICC representatives and senior officials of their organization the plans for public communications related to the outbreak;
- verifying data and information for communications products with their content experts/OICC representatives related to their respective jurisdiction; and
- coordinating input, feedback, and any concerns from their OICC representatives in a timely manner on all public communications products.
On an OCT teleconference or email, once a communications plan has been established by the OCT in collaboration with the OICC partners (via the OICC teleconference lead), the OCT lead will notify OICC partners by email of any decisions related to communications that have been discussed among OCT members. The OCT lead will circulate draft products and seek input to communications products from all OCT members. OCT members are responsible for seeking input to these products from their OICC representatives. Every effort will be made by the OCT lead to retrieve feedback from all OCT members before advancing products forward for final approval to the senior officials of the OICC lead organization. Once communications products are approved, the OCT chair will share final public communications products in advance of distribution, and outline the final approach and timing of product release to OCT members and the OICC lead for distribution to all OICC members.
In certain events and unforeseen situations it may not always be possible to coordinate all efforts for public communications and a partner organization may decide to take action related to public communications that was not agreed to or discussed within the OCT or the OICC. In this situation, the organization should advise all OICC partners through its OCT members and share draft messaging prior to releasing public communications products. Draft public messages must respect the confidentiality of information shared within the OICC, as outlined in the FIORP. Every effort should be made to inform all OICC partners of actions taken outside of an OICC teleconference.
7.10 OICC deactivation and outbreak conclusion
The OICC will evaluate all available evidence describing the progression of the outbreak in order to determine when response efforts can be concluded. The following considerations are made when deciding whether an OICC should be deactivated:
- There is consensus among the OICC partners that all avenues of investigation have been completed
- Pertinent investigation information has been shared and discussed among OICC partners
- The epidemiological investigation for all cases has concluded and there is no additional information expected
- The food safety investigation related to the outbreak investigation is complete and there is no additional information expected.
- The laboratory investigation for all cases is complete and there is no additional information expected
The OICC will review the status of the outbreak and come to a consensus on the OICC deactivation (i.e., general agreement among affected partners to deactivate). The OICC lead will then declare the deactivation of the OICC on the agreed-upon timelines. A notification is sent to the FIORP Duty Officers and P/T epidemiology and laboratory representatives informing them of the OICC deactivation.
The OICC lead should continue to monitor for ongoing cases that may need to be investigated for a period of time following the deactivation of the OICC to determine if they can be attributed to a particular source. If new information suggests that there is an ongoing risk, the timelines for deactivation can be reassessed and revised as necessary. If warranted, the OICC can also be reactivated with the consensus of the OICC partners.
The OICC will also collaboratively decide the criteria which must be met in order to declare the outbreak over. Three criteria that can be used to guide the decision to declare the end of an enteric illness outbreak are:
- The number of outbreak cases being reported to public health authorities has returned to baseline levels.
- The last time that individuals may have been exposed to the implicated source has been identified or estimated.
- Sufficient time has lapsed for potentially exposed individuals to become ill and be reported to investigating public health authorities.
Each enteric illness outbreak is unique, therefore it is essential to critically assess and adapt the criteria in the context of each outbreak. Depending on when these criteria are met, the date the outbreak is declared over may not always coincide with the OICC deactivation date. Once an outbreak investigation has been deactivated/closed, the foodborne illness outbreaks page on Canada.ca will be updated to reflect the final status of the event.
The OICC lead, with the assistance of agencies represented on the OICC, may prepare and circulate a final report and/or post a final summary on CNPHI’s Outbreak Summaries (Annex 2) to chronicle key events and findings from the outbreak investigation. Publication of the outbreak will be conducted in accordance with Guiding Principle X of the FIORP.
7.11 Post outbreak debrief
Post outbreak reviews may be conducted at the request of the OICC lead or any of the partners involved in the response. For a large outbreak involving multiple partners, a formal debriefing meeting is recommended. The outbreak debrief should be conducted in a timely manner after the resolution of the outbreak in order to benefit from the lessons learned. A representative from the OICC lead organization will chair the outbreak debrief unless otherwise agreed upon by the partners. Annex 11 provides a list of questions to be addressed.
The goals of the post-outbreak debrief should include, but are not limited to:
- confirmation of the outbreak cause;
- assessment of the effectiveness of outbreak control measures and any difficulties met in implementing the control measures;
- identification of the short- and long-term measures to prevent reoccurrence, such as new or revised policies or standards;
- evaluation of the collaborative response efforts, including communication and coordination between jurisdictions;
- clarification of resources, structural changes, or training needs to optimize future responses;
- identification of the necessary improvements or adjustments to the FIORP;
- discussion of any legal issues that may have arisen;
- assessment of a need for further scientific studies; and
- discussion of any knowledge mobilization activities.
It is the debrief chair’s responsibility to provide partners with a summary report of the debrief meeting. The partners may further distribute the report to other officials within their organizations who would benefit from the information.
8. Administrative review
PHAC will be the custodian of the FIORP and update the contact list on a quarterly basis. Under PHAC’s leadership, the FIORP will undergo a formal review process with F/P/T input every five years to keep the document up to date. Smaller-scale revisions will occur as necessary, to address issues identified during post outbreak debriefs, changes in organizational names or responsibilities, and to maintain up-to-date information regarding MOUs and information-sharing agreements as they are developed.
9. Emergency operations centre activation and incident command system
Most multi-jurisdictional foodborne illness outbreak investigations do not require the use of an incident command system (ICS) and activation of EOCs.
However, agencies may consider using such an approach for some public health emergencies, including foodborne illness outbreaks, to help coordinate the response. Agencies that are implementing an ICS will determine the types of events or outbreaks that will trigger the use of such a system and should incorporate these triggers into their agency’s response protocols. Agencies are responsible for notifying other investigative partners of their intent to utilize an ICS and activate their respective EOC(s). The OICC would continue to function as outlined in the FIORP.
10. List of acronyms
- ARC: Area Recall Coordinator (CFIA)
- CCMOH: Council of Chief Medical Officers of Health
- CFEZID: Centre for Foodborne, Environmental and Zoonotic Infectious Diseases (PHAC)
- CFIA: Canadian Food Inspection Agency
- CNPHI: Canadian Network for Public Health Intelligence
- EOC: Emergency Operations Centre
- ESPS: Enteric Surveillance and Population Studies (PHAC)
- FIORP: Foodborne Illness Outbreak Response Protocol
- FNIHB: First Nations and Inuit Health Branch (HC)
- F/P/T: Federal/Provincial/Territorial
- HC: Health Canada
- HRA: Health Risk Assessment
- ICS: Incident Command System
- IHR: International Health Regulations
- MOU: Memorandum of Understanding
- NML: National Microbiology Laboratory (PHAC)
- OCT: Outbreak Communications Team
- OFSR: Office of Food Safety and Recall (CFIA)
- OICC: Outbreak Investigation Coordinating Committee
- OMD: Outbreak Management Division (PHAC)
- PHAC: Public Health Agency of Canada
- P/T: Province or territory; provincial or territorial
- RCMP: Royal Canadian Mounted Police
- RRC: Regional Recall Coordinator (CFIA)
- TPP: Traveling Public Program (PHAC)
- WHO: World Health Organization
Annex 1
Outbreak investigation coordinating committee teleconference agenda template
| Agenda Item | Lead | Action Items | |
|---|---|---|---|
| 1 | Introductions and Participant Roll Call | ||
| 2 | Changes or Additions to the Agenda | ||
| 3 | National Epidemiologic Update (and international updates, if applicable)
|
||
| 4 | National Laboratory Update
|
||
| 5 | P/T Updates
|
||
| 6 | Food Safety Investigation Update
|
||
| 7 | Hypothesis Review
|
||
| 8 | Communications Updates
|
||
| 9 | Summary of Action Items/Next Steps | ||
| 10 | Next Teleconference Call |
Annex 2
Canadian Network for Public Health Intelligence (CNPHI)
The Canadian Network for Public Health Intelligence (CNPHI) is a secure online platform for applications and resources that facilitate communication and coordination through disease surveillance, intelligence exchange, research and response. Data can only be shared to public health stakeholders at the local/regional, P/T and national levels who have access to CNPHI and specific applications within CNPHI. CNPHI and its applications are not intended for public use. Only authorized users are granted access to application(s). CNPHI applications used in enteric outbreak investigations include Public Health Alerts, Outbreak Central and Outbreak Summaries.
Public Health Alerts
Public Health Alerts is an application on CNPHI that allows for the timely notification and/or dissemination of information between local/regional, P/T and national public health stakeholders. Users can select the target audience (i.e., single P/T, multiple P/Ts, national) to whom an email notification will be sent to advise that a new PHA has been posted. PHAs are used for case finding and to provide situational awareness on current national, P/T and local investigations.
Outbreak Central
Outbreak Central is an event board on CNPHI that is used by investigative partners to view and manage documents related to an outbreak investigation. These documents may include epi summaries, epi curves, event summaries, food frequency tables, maps and meeting minutes.
Outbreak Summaries
Outbreak Summaries is a secure, web-based application on CNPHI that provides a platform for local/regional, P/T and federal public health professionals to report standardized data from enteric illness outbreak investigations conducted in their respective jurisdictions. The application allows users to monitor trends in outbreaks across Canada and provides information for use in hypothesis generation, policy development and evaluation, and public health planning.
Annex 3
Assessment of the weight of epidemiological evidence
This guide introduces and describes the recommended data and criteria to consider when assessing the weight of the epidemiological evidence for a specific food as the source of an outbreak. An Epi Assessment template has been developed to assist investigators with the assessment (Section 2 of this Appendix). A standardized approach to the Epi Assessment using the template provided will facilitate Health Canada’s consideration of the epidemiological evidence in the HRA.
Epi Assessments need only present the evidence in enough detail to support their conclusion. Factors to consider are presented in the guide to assist with a comprehensive review of the evidence; responses need not be provided for all of these questions. If the information is already provided in another document (e.g., a comprehensive Epi Summary), the evidence need not be repeated; the reader can be directed to the document where the information can be found. A number of questions are also embedded in the guide for ease of completion.
Examples are included in this guide to assist with the completion of the Epi Assessment (Section 4 of this Appendix). In some instances, it may be apparent with few supporting statements that a food is the source of the outbreak illnesses (example 1). In other instances, a detailed review of the evidence may be required to come to a conclusion (example 2).
Details on epidemiological aAssessment criteria
A. Brief epidemiological summary:
- If a line list has not been provided throughout the course of the investigation, provide a line list of cases to help Health Canada to connect case-specific evidence from each of the three arms of the investigation (epidemiological, laboratory and food safety). The following variables, where available, should be considered for inclusion in the line list:
- Case ID, case confirmation status, age, sex, onset, and any relevant laboratory, food exposure and purchase information. Include sufficient detail to allow a thoughtful review of the evidence (e.g., whether the food was fresh or frozen, location and date of purchase, etc.).
- Provide an epidemiological summary describing the status, size and severity of the outbreak and the characteristics of the population involved to inform Health Canada’s risk assessment/risk characterization. Provide the following information where available:
- Case definitions
- Number of cases
- Severity of illness indicators: hospitalizations, complications (e.g., HUS), deaths
- Age and sex distributions
- Geographic distribution by P/T, regional/district/local health authority
- Time distribution: include an epidemic curve based on onset date, optionally an exposure curve or Gantt charts for restaurant clusters
- Other significant characteristics of population at risk (e.g., immuno-compromised, residents of long-term care homes, daycare population, etc.)
- Exposure details pertinent to the suspect food and other plausible exposures (include interpretation of cases that do not report eating the suspect food or cases that are considered outliers relative to the rest of the cases).
- Provide evidence that the outbreak described in the Epi Assessment represents a common source outbreak and not sporadic non-outbreak illnesses. Consider the following:
- Epidemiological indicators: are cases clustered in a specific and unusual time, place and/or population?
- Laboratory indicators: is the outbreak pathogen specific and unusual? Refer to Section B and consult microbiologist(s) on outbreak team.
- Based on a review of both epidemiological and laboratory data, is there evidence to indicate that this outbreak may involve multiple distinct pathogen sub-types?
- Are some cases meeting the outbreak case definition likely to be sporadic rather than outbreak illnesses?
B. Food under assessment:
Define the suspect food being assessed as the source of the outbreak. Consider different levels of specificity from general food type to specific product to lot codes if available from case interviews or other sources (e.g., shopper loyalty cards, inspection results).
- Suspect food: The primary focus of the assessment should be on the suspect food defined at a level of specificity for which there is most likely to be sufficient evidence to implicate it as the source of the outbreak. For example, if a majority of cases report a common brand of the food, focus the assessment on the particular brand-food combination. Alternatively, if a majority of cases report a food type with limited specific product information, focus the assessment on the food type, but also include the available product details.
- Other levels of specificity if applicable/information available: For many outbreaks, it will also be helpful to consider the evidence available for different levels of specificity of the food. More specific product details, even if limited at the time of the Epi Assessment, can be combined with evidence from the laboratory and food safety evidence in the HRA to build strong evidence. Throughout the relevant sections of the assessment (i.e., consistency, strength, consideration of alternate hypotheses), also consider the evidence for the food defined more specifically as the source of the outbreak.
- [e.g., food product]
- [e.g., brand, package type]
- [e.g., packager/distributer/manufacturer]
- [e.g., lot code/best before date]
- [e.g., packager/distributer/manufacturer]
- [e.g., brand, package type]
C. Assessment criteria:
Assess the evidence for the suspect food as the source of the outbreak based on each of the criteria below. Weight the evidence in support of the statement for each criterion as strong, moderate or weak.
- Strong – There is clear and convincing evidence in support of this criterion.
- Moderate – There is substantial evidence in support of this criterion, but additional evidence is required to make it strong.
- Weak – There is some evidence in support of this criterion, but there are important gaps.
Provide as much evidence as you feel is required to support the assessment of each criterion (e.g., brief responses, references to sections of the Epi Summary, detailed narrative – see Examples in Section 4 of the Appendix II).
1. Plausibility: The food is a plausible vehicle of infection.
Plausibility is usually assessed in the early stages of the outbreak investigation to develop hypotheses. Investigators typically refer to the history of the pathogen and past outbreaks for this purpose (e.g., outbreak reports, microbiological studies or surveillance of food, environment, and food-producing animals).
Consider the following:
- Is the food a known vehicle of infection for the outbreak pathogen?
- Has the pathogen been previously identified in the food type?
If the answer is yes to either of the above two questions, additional detail need not be provided. There is no need to document the evidence in detail or provide references to literature when the food in question is a well-known risk factor for infection with the outbreak pathogen. However, if the answer is no to both of these questions, any available evidence to support the food as a plausible vehicle of infection for this outbreak should be provided. For example, is the food item farmed/prepared/manufactured in a way that is similar to food items that have been implicated in past outbreaks or is it plausible that the pathogen could grow in the food (e.g., pH, amount of moisture, etc.). As part of the HRA process, Health Canada will conduct a detailed review of available science regarding growth and survival of the outbreak pathogen in the suspect or similar foods as necessary.
2. Temporality: Cases report eating the food within their period of exposure.
To establish causality, the exposure must precede the illness and should fall within a period of exposure consistent with the incubation period of the pathogen. This is typically accounted for during case interviews which focus on exposures within this timeframe.
Consider the following:
- What was the time period used to assess case exposures during interviews (e.g., 7 days, 10 days, etc.)?
- Do any cases only report eating the suspect food outside of this time period?
If exposures occur outside the expected timeframe for more than one or two cases, reasons for this should be explored (e.g., food is not the cause of the illness, unclear estimate of illness onset, difficulties recalling time of exposure, changes in pathogenesis, etc.).
3. Consistency: The distribution of cases in time and place is consistent with the shelf-life and distribution of the food.
Temporal and/or geographic clustering of cases that correlates with the availability and/or distribution of a particular product provides stronger evidence. Describe the distribution of cases relative to what is known about the distribution and shelf-life of the food.
Consider the following:
- What is the shelf-life of the suspect food?
- When was/is the suspect food available to consumers (i.e., consistently or variably over time/season)?
- Is the epidemic curve consistent with the shelf-life and availability of the suspect food?
- Where was/is the suspect food distributed?
- Have cases/case food purchases been identified where the suspect food was/is distributed?
- Are there any cases reporting exposure/purchases outside the time or area of the distribution of the suspect food?
Reasons for any inconsistencies should be explored (e.g., origin, pattern or frequency of food contamination, involvement of additional products containing suspect food as ingredient, etc.).
4. Consistency: The food exposure is consistently reported among cases.
The higher the number and proportion of cases who report eating the suspect food, the stronger the evidence. The evidence is also strengthened by demonstrating that the cases and/or case clusters reporting the exposure are otherwise unrelated (e.g., same food suspected based on two independent restaurant clusters). Summarise the number and proportion of cases exposed to the suspect food.
Consider the following:
- How many cases reported eating the food?
- What proportion of cases reported eating the food?
- Is the food exposure reported by a majority of cases in multiple independent clusters?
- What number and proportion of cases provided the same or similar more specific details on the origin of the food (e.g. common product details, purchase location, purchase dates, package type, brand, packager/distributor/manufacturer, lot code/best-before-date, etc.)?
- What are possible explanations for cases who report not eating the suspect food (e.g., hidden ingredient, difficulties noted in recalling food history, does eat suspect food but can’t recall eating it in exposure period, secondary transmission, cross-contamination, etc.)?
It is not necessary to duplicate evidence that will be submitted to Health Canada by the CFIA. However, public health and/or provincial/territorial food safety authorities may have information that may more specifically pinpoint the common food source (e.g., common product details, purchase location, purchase dates, package type, brand, packager/distributor/manufacturer, lot code/best-before-date, etc.). There may be too few cases reporting specific details to implicate a particular product, but this information is important as it will be combined with available traceback evidence in the HRA.
5. Strength of association: A higher than expected proportion of cases report the food exposure.
The gold standard epidemiologic evidence is a well-designed analytical study (e.g., case-control, cohort, case-case) demonstrating a strong and statistically significant association between the suspect food and the outbreak illnesses. In many instances, an analytical study is not feasible (e.g., due to time or resource constraints, difficulty identifying appropriate controls, difficulty ascertaining exposure because suspect food is an ingredient) and/or not warranted given the weight of other evidence available. Although less methodologically rigorous, investigators can also compare against the proportion of the general population that eats the food in question if these data exist (e.g., Foodbook, FoodNet Canada (formerly C-EnterNet), FoodNet USA, other).
Consider the following:
- Has an analytical study been conducted? If so, summarise design, results, limitations and conclusions or attach study documentation containing this information.
- Do data exist that estimate the proportion of the general population who eat the food or similar foods (e.g., Foodbook, FoodNet Canada (formerly C-EnterNet), FoodNet USA, other)? If so, indicate reference population data source and summarise the comparison, limitations and conclusions or attach documentation containing this information. Consider calculating the binomial probability of observing as many or more cases eating the suspect food by chance alone given the population food exposure frequency (e.g., using EpiInfo or Oregon Public Health’s binomial probability MS Excel worksheet) or conducting a statistical test for a difference in the proportions (e.g., chi-square test, z-test) if feasible.
- If no general population data exist, is there a reasonable estimate of the proportion of the general population who eat the food? If yes, describe the methodology used to make the estimate and summarise the comparison, limitations and conclusions or attach documentation containing this information.
6. Consideration of alternate explanations: Other plausible hypotheses have been adequately ruled out.
In establishing the suspect food as the source of the outbreak, it is critical to demonstrate that other plausible hypotheses have been explored and ruled out. Detailed case interviews, particularly at the outset of an outbreak investigation, help to ensure this, as well as thorough review of information for cases who report not eating the suspect food.
Consider the following:
- How many cases were interviewed regarding other exposure sources?
- What type of interview tool was used (e.g., open ended food history, routine enteric follow-up, extensive hypothesis-generating questionnaire, etc.)?
- Approximately how many other exposures were explored during case interviews?
- Have foods other than the suspect food, reported by a large (>50%) proportion of cases been ruled out? If so, briefly describe on what basis, particularly for foods that are also plausible vehicles of infection.
- Among cases that report not eating the suspect food, were there any other foods identified in common? If so, have these been ruled out as possible sources?
- Are there any cases with restricted diets or cases that never report eating certain foods?
- What foods may be commonly consumed at the same time or place as the suspect food or by the same people who eat the suspect food? Have these been ruled out as possible sources?
- If applicable, consider the possible role of food handlers in transmission via cross-contamination at implicated establishment(s) (e.g., restaurant, retail outlet) and of secondary person-to-person transmission. Interpret this information relative to the source of the outbreak, if applicable. This may explain transmission in some cases and can also explain observed inconsistencies in the evidence for a food as the source of the outbreak.
D. Epi assessment conclusion:
Based on an assessment of the epidemiological evidence, conclude that there is either strong evidence that the suspect food is the vehicle of infection for this outbreak or that there is a need for additional evidence.
Whenever possible, interpret the evidence for additional levels of specificity of the suspect food. Among cases reporting more specific details (e.g., common product details, purchase location, purchase dates, package type, brand, packager/distributor/manufacturer, lot code/best before date, etc.), what additional conclusions can be drawn? For example, there may be few cases that provided specific product details but of those who did, do the majority or a significant proportion indicate a common brand/supplier/etc.? Additionally, the purchase locations and time periods are critical for the traceback investigation and determining the scope of the implicated product.
Briefly highlight the most important gaps in the evidence to focus further investigation and evidence gathering as needed.
This information combined with findings from the laboratory, traceback and food safety investigation will inform timely and appropriate actions to control the outbreak.
E. Additional considerations:
Comment on any relevant food samples, such as samples collected from case homes, that have laboratory results pending or in which the outbreak pathogen has been detected. Assess the information using the criteria outlined in the Microbiological Stream of Evidence section, and through consultation with laboratory experts on the outbreak team as appropriate.
Outline any additional evidence (including circumstantial evidence, information from outlier cases, etc.), outstanding questions or pending information that may influence the assessment of the epidemiological evidence.
Epi assessment template
Important: Refer to the guide included in the previous section of this Annex for guidelines to complete this template.
Lead public health authority:
Version Date:
Version Time:
Outbreak number and title:
Note: Interpret and weigh the evidence for each criterion and summarise the supporting evidence below. Where the evidence is outlined and interpreted in the epidemiological summary, there is no need to duplicate it here. Refer the reader to the section of the epidemiological summary where the information can be found.
| Brief Epidemiological Summary (Refer to section of guide noted in the column at left): | ||
|---|---|---|
| A.1 | Has a line list been provided to investigative team members including Health Canada (e.g., case ID, case confirmation status, age, sex, onset, food exposure and purchase details)? | Yes No |
| A.2 | Has an epidemiological summary been provided to investigative team members including Health Canada? | Yes No |
| A.3 | There is substantial evidence that cases represent a common source outbreak. Briefly describe the evidence indicating cases represent a common source outbreak: |
|
| Food Under Assessment: | ||
| B.1 | Suspect food: | |
| B.2 | Other levels of specificity if applicable/information available (e.g., common product details, purchase location, purchase dates, package type, brand, packager/distributor/manufacturer, lot code/best before date, etc.):
|
|
| Epidemiological Assessment Criteria and Considerations | ||
| C.1 | Plausibility: The food is a plausible vehicle of infection. Provide supporting evidence: |
Strong Moderate Weak |
|
Yes No |
|
|
Yes No |
|
| If yes to either of the above two questions, no further explanation is required.
If ‘No’ to both of the above questions, provide any available evidence in support of the food as a plausible vehicle of infection: |
||
| C.2 | Temporality: Cases report eating the food within their period of exposure.
Provide supporting evidence: |
Strong Moderate Weak |
|
||
|
Yes No |
|
| If Yes, please explain. | ||
| C.3 | Consistency: The distribution of cases in time and place is consistent with the shelf-life and distribution of the food. | Strong Moderate Weak |
| Provide supporting evidence: | ||
| C.4 | Consistency: The food exposure is consistently reported among cases. | Strong Moderate Weak |
| Provide supporting evidence: | ||
| C.5 | Strength of association: A higher than expected proportion of cases report the food exposure. Provide supporting evidence: |
Strong Moderate Weak |
|
Yes No |
|
|
Yes No |
|
| If ‘Yes’, provide further details. If ‘No’ provide any available evidence that suggests a higher than expected proportion of cases report the food exposure: | ||
| C.6 | Consideration of alternate explanations: Other plausible hypotheses have been adequately ruled out. | Strong Moderate Weak |
| Provide supporting evidence: | ||
| Conclusion | ||
| D | Is there strong epidemiological evidence that the [suspect food] is the vehicle of infection for this outbreak? | Yes Additional evidence needed |
| State any additional conclusions that can be made regarding specific details of the suspect food (e.g., product, purchase locations, purchase time periods, origin of the food): | ||
| Briefly highlight any important gaps in the evidence: | ||
| E | Additional considerations: | |
Annex 4
Botulism Reference Service for Canada
The management of a suspected botulism case involves healthcare professionals, and P/T and federal public health officials. The federal management involves Health Canada’s Botulism References Service (BRS) for Canada and Special Access Program (SAP). For further information on the treatment of botulism and submission of samples to the BRS, consult Health Canada’s Botulism – Guide for Healthcare Professionals.
The BRS for Canada, established in 1974, provides the following support:
- Assists physicians and P/T officials when botulism is suspected;
- Examines suspect foods and clinical specimens submitted for analysis;
- Rapidly alerts responsible agencies when commercial foods are involved;
- Maintains reference cultures of Clostridium botulinum; and
- Liaises with centres that have similar interests and responsibilities in Canada and abroad.
Botulism antitoxin and immune globulin are not approved for sale in Canada; specified quantities are currently only available via authorization through Health Canada’s Special Access Programme (SAP). The procedure for healthcare workers and facilities/organizations providing healthcare, however, varies between P/Ts. Please check with the office of the Chief Medical Officer of Health for the P/T reporting requirements. Symptoms of foodborne botulism include ptosis, visual disturbance, vomiting and diarrhea, dry mouth and sore throat, followed by descending symmetrical flaccid paralysis in an alert, febrile person. Similar symptoms are associated with wound botulism, but vomiting does not occur. The earliest signs observed in infant botulism are related to the paralysis of the bulbar musculature and include difficulty feeding, poor sucking and swallowing, difficulty managing secretions, diminished facial expression and altered crying. This is followed by progressive, descending weakness/hyptonia (“floppy baby”). When health care providers take a careful history, parents also often recall a change in stooling pattern or constipation. Although constipation is one of the most common symptoms, it may be overlooked as an early symptom of infant botulism.
In cases of foodborne or wound botulism, a specific antitoxin is administered as soon as possible. In infant botulism, a human botulism immune globulin (known by the trade name BabyBIG®) exists, and is a safe, highly efficacious treatment when administered early in the infant’s hospital course. For all types of botulism, meticulous supportive care and accessibility to respiratory support are essential.
When botulism is suspected, a member of the BRS should be called immediately, day or night. The possible diagnosis of botulism will be validated by discussing the clinical presentation of the suspect case of botulism, and plans for transporting suspect food and clinical specimens to Ottawa for laboratory analysis can be finalized. Clinical specimens must be obtained prior to administrating botulism antitoxin. The food samples may be leftovers or unopened containers. When commercial foods are involved, it is important to retrieve the label, the manufacturer’s lot number, codes embossed on the can or package, etc.
Suitable clinical specimens for analysis include:
- fecal samples (approximately 10 g)
- enema fluid
- gastric contents (adjusted to approximately pH 6.0 with 1N NaOH, if possible);
- serum (from 20 ml of blood collected BEFORE administration of antitoxin); and
- For suspected infant botulism, the essential material for analysis is the infant’s feces. If necessary, the soiled parts of diapers, a rectal swab, 2 ml of serum or a combination of samples may be submitted.
After collecting the sample, but prior to shipping, ensure that the sample is kept in the refrigerator at 4°C. Ship specimens in a watertight primary receptacle, in a watertight secondary container, with sufficient absorbent material between the two containers to absorb the entire contents of the primary receptacle. The preferred method of preserving the material is by cooling rather than freezing (i.e., by including commercial cooling packs in the parcel). After the specimen is shipped, inform BRS of the expected delivery time. In urgent cases, the parcels are picked up immediately upon arrival, usually at the airport.
Botulism Reference Service:
Chair (613-957-0902)
After-hours 613-296-1139)
Analyst (613-957-0885)
Health Products and Food Branch
Health Canada
Banting Research Centre
251 Sir Frederick Banting Driveway
Ottawa, Ontario, K1A 0K9
Postal Locator 2204E
If the samples are from BC or if the Botulism Reference Service is not available, the BC Centre for Disease Control (BCCDC) labs will provide backup capacity for botulism testing as described in the business continuity plan for the Botulism Reference Service.
Contact information for the Botulism Testing at BCCDC:
Contact the BCPHMRL Medical Microbiologist on call at 604-661-7033 for regular and after hours.
Technical consultation is available during regular hours through:
Brian Auk (604-707-2608)
Section Head
Environmental Microbiology Laboratory
Botulism antitoxin
There are four products considered for access through the SAP: 1) Botulism Antitoxin Type AB and Type E, accessed from the Butantan Institute in Brazil; 2) Novartis Trivalent Types ABE; 3) BabyBIG®, Botulism Immune Globulin Intravenous (Human) (BIG-IV) for pediatric patients under the age of 1, accessed from the Infant Botulism Treatment and Prevention Program (IBTPP) at the California Department of Public Health (CDPH); and 4) NP-018 (heptavalent) Types A to G from Cangene Corporation. Requests for any of these products require the submission of an SAP request form by a practitioner. In addition to the submission of an SAP request form for access to BabyBIG®, the IBTPP requires practitioners to complete additional forms. This is part of its internal process for considering and providing access to BabyBIG® for Canadian practitioners. The SAP considers requests from practitioners for access to non-marketed drugs for treatment, diagnosis, or prevention of serious or life-threatening conditions when conventional therapies have been considered and ruled out, have failed, are unsuitable, and/or are unavailable. The regulatory authority supporting the program is discretionary and a decision to authorize or deny a request is made on a case-by-case basis by taking into consideration the nature of the medical emergency, the availability of marketed alternatives, and the information provided in support of the request regarding the use, safety, and efficacy of the drug.
For more information on the SAP and for a copy of the request forms, please refer to the SAP website. The main contact number for SAP is 613-941-2108. If the case presents on a weeknight, weekend or holiday, the SAP on-call officer can be reached by telephone at 613-941-2108 (press 0). For further product information, please contact the respective manufacturers. Their phone numbers and website addresses are provided. Butantan Institute at 011-55-11-37263816, Novartis Canada, the Infant Botulism Treatment and Prevention Program, CDPH, at 1-510-231-7600 (24/7), and Cangene Corporation 416-948-8285.
Annex 5
Listeriosis Reference Service for Canada
The Listeriosis Reference Centre (LRC) for Canada is a joint responsibility of the Bureau of Microbial Hazards (BMH) within the Food Directorate in Ottawa and the National Microbiology Laboratory (NML) in Winnipeg. Established in 2001, the LRC has the following objectives:
- to assist physicians and P/T public health authorities when foodborne listeriosis is suspected;
- to examine suspect foods and clinical specimens submitted for analysis;
- to perform identification and typing using pulsed-field gel electrophoresis, ribotyping, serotyping of O-antigens and H-antigens and next generation typing schemes (e.g. whole genome sequencing and bioinformatics);
- to rapidly alert responsible agencies when commercial foods are involved;
- to maintain reference cultures of Listeria monocytogenes;
- to liaise with organizations with similar interests/responsibilities in Canada and abroad; and
- to reduce the number of foodborne listeriosis cases and hence the disease burden due to this pathogen in Canada.
Listeria monocytogenes is a psychotropic organism, capable of growing at refrigeration temperatures and is of concern in extended shelf life refrigerated, ready-to-eat foods. The ability of the organism to grow over a wide temperature range, and in the presence or absence of O2, enables it to multiply in many environments. It is considered that as much as 80% to 90% of human listeriosis cases are linked to the ingestion of contaminated food. The majority of human cases occur in high-risk individuals, including organ transplant recipients, patients with AIDS and HIV-infected individuals, pregnant women, cancer patients, and the elderly. Infection in pregnant women usually results in a mild flu-like illness. Although it can occur at any time, infection of the fetus usually occurs in the third trimester, and can lead to abortion, stillbirth, or delivery of an acutely ill baby. However, it presents most commonly as an infection causing pre-term labour. The major manifestations of the disease in non-perinatal cases are septicaemia and/or meningitis. In the USA, L. monocytogenes is the fifth most common cause of bacterial meningitis, and the most common organism causing meningitis in patients with underlying malignancies. It is the third leading cause of bacterial neonatal meningitis in the UK. Central nervous system cases of L. monocytogenes are associated with a high mortality rate, and neurological sequelae can occur among survivors.
When foodborne listeriosis is suspected, a member of the LRC should be notified. Plans for transporting suspect food to the LRC for laboratory analysis should be arranged. Food samples from the refrigerator may be leftovers or unopened containers. When commercial foods are involved, it is important to retrieve the label, manufacturer's lot number, codes embossed on the can or package, etc. In addition to clinical, environmental and food specimens, the LRC can receive isolates for identification and molecular characterization. Shipping of specimens shall be down by a TDG (transport of dangerous goods)-certified individual in accordance with TDG regulations. LRC serves to coordinate activities and provide accompanying information on incoming isolates. The information collected in this database will be enhanced with the addition of isolates collected from L. monocytogenes-contaminated foods involved in recalls.
The LRC will also provide P/T epidemiologists and public health labs with a central reference lab capable of isolating L. monocytogenes from food, clinical, and environmental samples associated with sporadic cases and outbreaks of foodborne listeriosis. The LRC also participates in Listeria research projects promoting public health and is available for consultation on Listeria-related activities. The LRC is a member of the World Health Organization (WHO) Collaboration Centre for Listeria, contributing to the WHO’s efforts in the international surveillance of listeriosis, improving knowledge on epidemiology of listeriosis, its microbiological and clinical features, contribution to WHO/FAO risk analysis, antimicrobial resistance and in capacity building. L.monocytogenes is included as one of the organisms in PulseNet Canada.
LRC Chairs and contact information:
Dr.Franco Pagotto
(office 613-957-0895)
Health Products and Food Branch
Health Canada
251 Sir Frederick Banting Driveway
Ottawa, Ontario, K1A 0K9
Postal Locator 2204E
franco.pagotto@hc-sc.gc.ca
Dr. Celine Nadon
(office 204-789-5037)
Enteric Diseases Program
National Microbiology Laboratory
Public Health Agency of Canada
1015 Arlington Street
Winnipeg, Manitoba, R3E 3R2
NML.Enterics@phac-aspc.gc.ca
Annex 6
Food Virology Reference Centre for Canada
The Food Virology Reference Centre (FVRC) for Canada, established in 2007, is located in the Bureau of Microbial Hazards (BMH) in the Food Directorate (Ottawa), and deals with food-related viral agents. The Virology Reference Centre in the National Microbiology Laboratory (NML) in Winnipeg deals with viral agents in clinical samples. A joint agreement has been established between BMH and NML whereby both centres work jointly to encompass both clinical and food-related viral agents.
The FVRC has the following objectives:
- to develop and maintain standard methods to extract viruses from foods;
- to examine suspect foods and clinical specimens submitted for analysis;
- to rapidly alert responsible agencies when commercial foods are involved;
- to maintain reference collections of enteric viruses (samples, cultures); and
- to maintain liaison with centres that have similar interests and responsibilities in Canada and abroad.
Symptoms of norovirus infection include nausea, abdominal cramps, vomiting, diarrhea and fever. Outbreaks of norovirus can be identified with 99% specificity using all four of the Kaplan criteria, namely,
- Vomiting in more than half of the affected persons;
- Mean or median incubation period of 24–48 hours;
- Mean or median duration of illness of 12–60 hours; and
- No bacterial pathogen in stool culture.
Symptoms of hepatitis A virus infection include nausea, anorexia, fever, malaise, or abdominal pain and jaundice. Hepatitis A diagnosis is confirmed by a positive serologic test for immunoglobulin M (IgM) antibody to hepatitis A virus, or occurs in a person who has an epidemiologic link with a person who has laboratory-confirmed hepatitis A.
When foods are suspected as a cause of norovirus or hepatitis A virus outbreaks, a member of the FVRC is available, day or night, to provide support as requested for the investigation. Validated food testing methods can be provided to outside laboratories, or plans for transporting suspect food and clinical specimens to Ottawa for laboratory analysis can be finalized. FVRC is well equipped to analyze suspected food samples, but it also has the capacity to genotype clinical specimens to support the epidemiological association of a food item with the outbreak cases. When commercial foods are involved, it is important to retrieve the label, the manufacturer’s lot number, and codes embossed on the can or package. Suitable clinical specimens for analysis include fecal samples (approximately 5 g) or enema fluid and vomitus. If necessary, soiled parts of diapers may be submitted.
For safe shipment, the specimens should be in a watertight primary receptacle, in a watertight secondary container, with sufficient absorbent material between the two containers to absorb the entire contents of the primary receptacle. The preferred method of preserving the material during shipment is by cooling rather than freezing, i.e. by including commercial cooling packs in the parcel.
Food Virology Reference Centre:
N. Corneau
(office 613-954-7728)
O. Mykytczuk, Analyst
(office 613-957-0909, cell 613-265-9654)
Health Products and Food Branch
Health Canada
251 Sir Frederick Banting Driveway
Ottawa, Ontario K1A 0K9
Postal Locator 2204E.
The Food Virology Reference Centre Is Co-chaired By:
Dr. Tim Booth, Director
(office: 204-789-2022),
Viral Diseases National Microbiology Laboratory
Public Health Agency of Canada
1015 Arlington Street
Winnipeg, Manitoba R3E 3R2.
Annex 7
Canadian Food Inspection Agency - Laboratory Testing
Roles and Responsibilities
The Canadian Food Inspection Agency (CFIA) Science Branch has a mandate to provide scientific leadership, advice, and laboratory services to contribute to an effective science-based organization.
The CFIA’s food laboratories deliver both microbiology and chemistry testing services. The laboratory analytical services are primarily focussed on the analysis of food and food establishment environmental samples normally provided by the CFIA Operations Branch. These samples are either from routine monitoring plans or from complaints and investigations. The CFIA will also provide testing support to other agencies and departments on a case-by-case basis during investigations.
Available resources
The CFIA laboratories work under ISO Quality Systems accredited to ISO/IEC 17025:2005 and have a wide array of analytical technologies that ensure the validity of test results.
Food Microbiology Laboratory services
Methodology used in the CFIA food microbiology laboratories must meet the CFIA requirements for regulatory testing and be equivalent to methods used to set the standards (set by HC) for various food commodities.
The CFIA has a network of eight laboratories across the country with capability for testing most foodborne bacterial pathogens, indicator organisms, viruses, and parasites. In addition to these eight laboratories, the CFIA has a newly acquired PFGE PulseNet laboratory.
The CFIA has research capabilities in the areas of virus, parasite, and bacterial pathogens.
Food Chemistry Laboratory services
Methods of analysis used in the CFIA food chemistry laboratories may be official methods prescribed by regulations, official methods validated and published by outside organizations such as AOAC International, ISO, IUPAC, and others, or may be methods validated by the CFIA laboratories.
Chemistry testing also makes use of a network of eight laboratories across Canada. Additionally, for the National Chemical Residue Monitoring Program, the CFIA augments laboratory testing capacity by utilizing the services of five Canadian private sector ISO accredited laboratories. The chemistry laboratories are generally specialized in specific disciplines, including but not limited to, pesticide residues, heavy metals and mercury, veterinary drug residues, marine toxins, mycotoxins, food allergens, environmental contaminants (dioxins, furans, PCBs and PAHs), food additives (approved and unapproved), food nutrition, and volatile organics.
Contribution to outbreak investigation and response
The CFIA laboratories provide scientific advice and testing capacity for microbiological and chemical contaminants in food and food environmental samples.
The CFIA laboratories also provide PFGE testing for Salmonella, Listeria, E. coli O157 and Shigella isolates to industry when requested by the Office of Food Safety and Recall or commodity programs.
Annex 8
Surveillance for Foodborne Illness
National Enteric Surveillance Program
The National Enteric Surveillance Program (NESP) is designed to provide timely analysis and reporting of laboratory-confirmed enteric disease cases in Canada. The program has been in operation since April 1997 and provides weekly reports to stakeholders across the country. NESP attempts to provide an up-to-date picture of the current status of major enteric infectious diseases in the human population such as Verotoxigenic E. coli, Listeria monocytogenes, Salmonella, Shigella, Campylobacter, Vibrio, Yersinia and more recently intestinal parasitic organisms, such as Giardia, Cryptosporidium, Entamoeba and Cyclospora as well as enteric viruses including Hepatitis A, Norovirus and Rotavirus.
The national incidence of foodborne pathogens is actively collected and reported by the NESP. The provincial public health laboratories provide the National Microbiology Laboratory (NML) with weekly aggregate data of their laboratory-confirmed isolations of foodborne pathogens. These laboratory characterizations are performed within their local jurisdiction or with the support by the NML reference laboratory services. The national and P/T trends of emerging and priority infectious diseases are then analyzed and communicated weekly back to P/T partners, and these data provide the basis for weekly discussions between federal laboratories and epidemiologists involved in food and water safety. NESP provides surveillance data that contributes to the detection of potential outbreak events, which leads to further laboratory characterization (fingerprinting) of those pathogens by PulseNet Canada to positively identify clusters which triggers the need to respond to possible outbreaks of foodborne disease.
PulseNet Canada
PulseNet Canada is a national network of F/P/T public health and food regulatory agency laboratories coordinated by the NML. Participating laboratories conduct standardized molecular subtyping of foodborne disease pathogens (DNA fingerprinting) and maintain centrally accessible databases of patterns. PulseNet also functions as a communication hub for laboratories involved in food and foodborne disease monitoring.
PulseNet Canada represents an active partnership between PHAC, the CFIA, Health Canada and the provincial public health laboratories (as represented by the Canadian Public Health Laboratory Network (CPHLN)) with the goals of real-time detection and response to foodborne outbreaks. Through coordinated action and communication between clinical and food laboratories, it is possible to collect laboratory evidence for clusters of human disease with a linkage to foodborne pathogens. PHAC epidemiologists provide joint response capabilities along with epidemiologic evidence for the identification of foodborne disease clusters.
PulseNet Canada member laboratories perform fingerprinting of pathogen isolates and then submits the resulting data and associated pathogen information to the NML. Centralized analysis occurs at the NML in partnership with the submitting laboratory, and real-time communication to all PulseNet Canada members then occurs on the Canadian Laboratory Surveillance Network (CLSN) discussion boards, hosted by CNPHI. When a cluster of human illness is identified within a single P/T, the respective provincial laboratory will post the information to the PulseNet Canada discussion board to inform the other network members of the emerging issue. If the PulseNet Canada database managers at the NML identify a multi-provincial cluster of isolates with the same fingerprint, they will lead the posting of any multi-provincial cluster. It is also possible for any PulseNet Canada member to submit pathogen isolates to the NML for DNA fingerprinting. The data will then be returned for use by the member, and any resulting analysis can be posted using the same protocols described above. Notably, the CFIA contributes real-time postings to the PulseNet Canada discussion board after it isolates and fingerprints foodborne pathogens, and this supports attribution between contaminated food and resulting human illnesses. Furthermore, if the CFIA identifies a foodborne contaminant but no human-clinical matches are observed at the time, this still provides the opportunity for P/T laboratories to be vigilant for this pathogen.
Since pathogen-specific surveillance does not depend on geographic clustering, it is more sensitive to detection of widespread, low-level contamination events than are outbreaks reported by notification. Outbreaks detected by this method tend to span multiple jurisdictions.
The uses of molecular subtyping and PulseNet Canada have improved the timeliness of detection of geographically dispersed foodborne disease outbreaks that span multiple jurisdictions. The timely communication of information regarding clusters of matching PFGE to F/P/T stakeholders facilitates the identification of relevant information from the local or P/T level and initiates a real-time, multi-jurisdictional discussion regarding the laboratory findings.
Annex 9
Laboratory capability for food and clinical microbiology
Federal Laboratory capability for food and clinical microbiology
| Detection and Isolation of Microorganisms from Food and Environmental Samples | Detection and Isolation of Microorganisms from Clinical Specimens | Identification and Characterization of Microorganisms (any source) | |
|---|---|---|---|
| Canadian Food Inspection Agency (CFIA) | Salmonella, Listeria, E. coli O157:H7, non-O157 VTEC, Shigella, indicator organisms, Staphylococcus toxin, Bacillus cereus, Clostridium perfringens, Pseudomonas aeruginosa, Campylobacter, Yersinia, Enterobacter sakazakii (Cronobacter), Vibrio, parasites, viruses. Also commercial sterility/canning integrity and extraneous matter |
Pulsed field gel electrophoresis (PFGE) (Listeria, Salmonella, E.coli, Shigella) | |
| Health Canada (HC) | Salmonella, Listeria, E. coli O157:H7, non-O157 VTEC, Shigella, indicator organisms, Clostridium perfringens, C. botulinum, Aeromonas hydrophila, Campylobacter, Enterobacter sakazakii (Cronobacter), Vibrio, parasites, viruses | Salmonella, Listeria, E. coli O157:H7, non-O157 VTEC, Shigella, indicator organisms, Clostridium perfringens, C. botulinum, Aeromonas hydrophila, Campylobacter, Enterobacter sakazakii (Cronobacter), Vibrio, parasites, viruses | Phenotypic and genotypic characterization of foodborne bacteria, viruses, parasites. Includes PFGE (for Listeria, Salmonella, E. coli and Shigella), polymerase chain reaction (PCR) and real-time PCR, DNA chip microarray for noroviruses and Campylobacter, ribotyping, dedicated sequence analysis, electron microscopy, and rapid method development |
| Public Health Agency of Canada (PHAC) | Bacteria: not offered as a routine service, but can assist as needed upon request Viruses: not offered as a routine service, but can assist as needed upon request |
Bacteria: primarily performed at the local or P/T level. PHAC can assist as needed upon request.
Viruses: enterovirus, rotavirus (culture, molecular tests or electron microscopy), norovirus, astrovirus (molecular tests or electron microscopy) |
All foodborne/enteric bacteria: identification, speciation, serotyping, phage typing, PFGE, antimicrobial resistance testing, toxin typing, additional molecular subtyping, sequencing, rapid method development Viruses: PCR and/or sequencing of rotavirus, norovirus, enterovirus, hepatitis A |
Note: Some tests in federal laboratories are accredited to ISO/IEC 17025. For further information, contact the laboratory.
Surge capacity procedures
Federal laboratories provide surge capacity where capability exists, upon request or as described by existing MOUs. To request surge capacity, contact the following:
Public Health Agency of Canada
Dr. Celine Nadon
Tel: 204-784-7507
Celine_Nadon@phac-aspc.gc.ca
Health Canada
Denise MacGillivray
Tel: 613-957-0881
denise.macgillivray@hc-sc.gc.ca
Canadian Food Inspection Agency
Annie Locas
Tel: 613-410-6519
annie.locas@inspection.gc.ca
Figure 3 - Microbiological data flow between Federal Laboratories
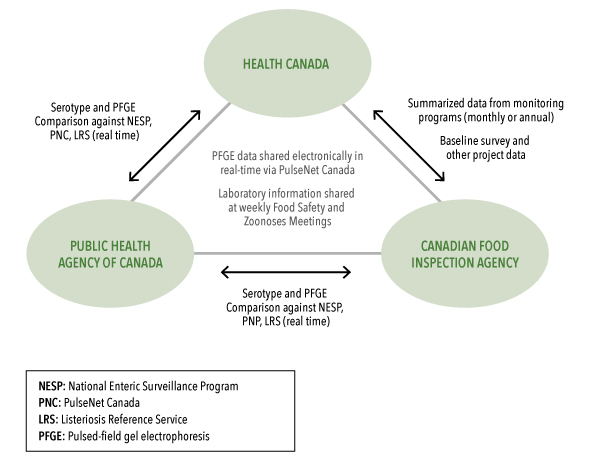
Figure 2: Microbiological Data Flow between Federal Laboratories
This figure represents the microbiological flow that occurs between federal laboratories.
Health Canada, the Public Health Agency of Canada, and the Canadian Food Inspection Agency each work together to share and compare data. The points of contact are the laboratory groups of each agency. Data is shared electronically in real time via PulseNet Canada, and laboratory information is shared at weekly food safety and zoonoses meetings.
Specifically, serotype and PFGE (pulsed-field gel electrophoresis) are compared against the National Enteric Surveillance Program (NESP), PulseNet Canada (PNC), and the Listeriosis Reference Service (LRS) between PHAC and Health Canada, and between PHAC and CFIA. Data from monthly or annual monitoring programs, baseline surveys, and other projects data are shared between Health Canada and the CFIA.
Provincial laboratory capability for food and clinical microbiology
| Detection and Isolation of Microorganisms from Food and Environmental Samples | Detection and Isolation of Microorganisms from Clinical Specimens | Identification and Characterization of Microorganisms (any source) | |
|---|---|---|---|
| Hospital laboratories | None | Isolation and identification of many common enteric human pathogens: bacteria, fungi, parasites, rotavirus | None |
| Provincial Public Health Laboratory | Salmonella, Shigella, E. coli O157 VT+, E. coli non-O157 VT+, Staphylococcus aureus, Bacillus cereus, Campylobacter, Yersinia, Pseudomonas aeruginosa, Clostridium perfringens, Listeria, Aeromonas, Plesiomonas, Vibrio | Salmonella, Shigella, E. coli O157 VT+, E. coli non-O157 VT+, Staphylococcus aureus, Bacillus cereus, Campylobacter, Yersinia, Pseudomonas aeruginosa, Clostridium perfringens, Listeria, Aeromonas, Pleisomonas, norovirus, Vibrio | Serotyping / Salmonella / Shigella VT detection/identification E. coli O157 |
| Detection and Isolation of Microorganisms from Food and Environmental Samples | Detection and Isolation of Organisms from Clinical Specimens | Identification and Characterization of Microorganisms (any source) |
|
|---|---|---|---|
| PEI Provincial Laboratory (Queen Elizabeth Hospital) | Typical bacterial pathogens present in stools (enteric pathogens): E coli, Salmonella, Shigella, Campylobacter, Vibrio species, Yersinia species (not typically done but can do if specifically requested). Other pathogens that the lab can detect from non-fecal samples include Listeria, Clostridium perfringens and Enterobacter sakazakii. Viruses: rotavirus, adenovirus, norovirus |
Queen Elizabeth Hospital identifies these organisms to the genus level (except for Campylobacter) using phenotypic methods and sends these to the National Microbiology Laboratory (NML) for speciation and genotyping (except for Campylobacter). Rotavirus and adenovirus are done using a rapid immunochromatographic test. Currently, Queen Elizabeth Hospital sends out stools to be tested at Halifax for norovirus via PCR but that could change in the near future, whereby the hospital will do this in-house instead. |
|
| PEI Food Technology Centre | E. coli O157:H7, Clostridium spp. (not C. botulinum), Salmonella spp. (no serotyping performed), Campylobacter spp. (including C jejuni, C. coli, C.hyointestinalis and C. lari), Staphylococcus aureus (we can provide toxin testing), B. cereus (and other species), Aeromonas spp. and Listeria spp. (incl. all 7 species) Water samples: Cryptosporidium, Giardia |
With respect to speciating some samples (i.e. B. cereus) and performing toxin testing (i.e. Staphylococcus), further confirmation steps are necessary and P.E.I. Food Technology Centre may not have the required media/tests readily on hand for that specific organism. Usually P.E.I. Food Technology Centre can order the necessary media/test and receive the order within 1–2 days. This should not be an issue as PEI Food Technology Centre would order what media it did not have on hand at the beginning of testing. Therefore, when PEI Food Technology Centre reached the confirmation steps, it would be equipped to do so. |
| Detection and Isolation of Microorganisms from Food and Environmental Samples | Detection and Isolation of Microorganisms from Clinical Samples | Identification and Characterization of Microorganisms (any source) | |
|---|---|---|---|
| Anchor Laboratories (Capital District Health Authority and IWK Health Centre) of the Provincial Public Health Laboratory Network | Food samples are not tested at the Provincial Public Health Laboratories Network (PPHLN) anchor laboratories. They are referred to the CFIA Routine testing of potable water and wastewaters: coliforms, E. coli, enterococci |
The anchor laboratories are tertiary care clinical microbiology laboratories and process all standard clinical samples Organisms sought in enteric testing: Salmonella spp., Shigella spp., Campylobacter spp., Yersinia spp., E. coli O157 EHEC (outbreak only, testing at IWK Health Centre), Vibrio spp. (selected cases), Plesiomonas shigelloides From non-enteric sites: Listeria monocytogenes Other biosafety level 2 organisms Biosafety level 3 organisms: Mycobacterium tuberculosis Virology diagnostic services Molecular diagnostic services, including influenza A, B, respiratory syncytial virus (RSV), herpes, etc. 16S rDNA detection/ identification testing Parasitology diagnostic services Mycology diagnostic services |
Biochemical and molecular identification MALDI-TOF PulseNet North – isolates referred to NML for testing NESP participant Limited Salmonella serotyping, Shigella serotyping, Neisseria meningitides serotyping |
| Detection and Isolation of Microorganisms from Food and Environmental Samples | Detection and Isolation of Microorganisms from Clinical Specimens | Identification and Characterization of Microorganisms (any source) | |
|---|---|---|---|
| NB Department of Agriculture, Aquaculture and Fisheries (DAAF) Veterinary Laboratory | Currently operates a basic microbiology laboratory with the capability of isolating microbial pathogens from clinical and environmental samples | Capacity to detect and identify parasitic agents such as Giardia and Cryptosporidium | Enhanced recovery techniques for Salmonella and Listeria Salmonella, once confirmed, is sent to PHAC reference laboratory for serotyping and phage typing |
| NB DAAF Fish Health Laboratory | Level 2 containment lab with Class 2 biosafety hoods. Microbiology and PCR labs could easily be modified to handle any environmental, clinical or food testing | Normally provides detection and identification on marine bacterial pathogens such as Vibrio and Aeromonas by biochemical and serological testing and by PCR methods for viral and parasitic organisms | |
| NB Region 2 reference microbiology laboratory | Salmonella, Shigella, E. coli O:157, Yersinia, Campylobacter, Aeromonas, Plesiomonas, Vibrio, viruses, parasites | Serotyping (Salmonella, Shigella, E. coli O:157) PFGE (Salmonella, E. coli O:157) Biochemical analysis (Salmonella, Shigella, E. coli O:157, Yersinia, Campylobacter, Aeromonas, Plesiomonas, Vibrio) |
|
| NB Department of Environment Laboratory | Detection and enumeration of total coliforms, E. coli, Enterococcus spp., fecal coliforms, and Pseudomonas spp. from water samples only | N/A | Limited biochemical analysis of gram-negative organisms (using API 20E technology) |
| Detection and Isolation of Microorganisms in Food and Environmental Samples | Detection and Isolation of Microorganisms in Clinical Samples | Identification and Characterization of Microorganisms (any source) |
|
|---|---|---|---|
| Ministère de l’Agriculture, des Pêcheries et de l’Alimentation du Québec (MAPAQ) Direction d’inspection des aliments (food inspection branch) Laboratoire d’expertises et d’analyses alimentaires (expert food analysis laboratory) |
Salmonella, Listeria, E. coli O157:H7, E. coli non-O157 VTEC, indicator organisms, toxins of: Staphylococcus, Bacillus cereus, Clostridium perfringens, Pseudomonas aeruginosa, Campylobacter, viruses (norovirus) Also sterility / commercial packaging integrity and foreign matter |
||
| Ministère de la Santé et des Services sociaux (MSSS) Laboratoire de santé publique du Québec (Quebec public health laboratory) |
Note: Bacteria isolation and detection is usually carried out in hospital laboratories Microscopic examinations to identify parasites in stools (Giardia, Cryptosporidium, Cyclospora) Detection of gastroenteritis viruses using a nucleic acid amplification test (NAAT) (norovirus) or electronic microscopy (sapovirus, rotavirus, astrovirus, adenovirus) |
Confirmation of bacterial identification: Salmonella, Listeria, E. coli O157:H7, non-O157 VTEC (via PHAC), Shigella, Staphylococcus, Bacillus cereus, Clostridium perfringens, Pseudomonas aeruginosa, Campylobacter, Yersinia, Enterobacter sakazakii (Cronobacter), Vibrio Detection of botulinum toxins (via PHAC) Molecular epidemiology using PFGE (Listeria, Salmonella, E. coli O157:H7, Shigella, Campylobacter, Pseudomonas, etc.) |
The Laboratory of Food Expertise and Analysis (LEAA) also has chemical expertise for the identification of foreign materials, animal species, food allergens and intolerances, pesticides, heavy metals, veterinary drug residues, industrial contaminants, natural toxins (mycotoxins, patulin, marine toxins, etc), radionuclides, and contaminants.
The MSSS Centre de toxicologie du Québec (CTQ) can contribute to the identification of chemical toxins that contaminate food. The laboratory is open seven days a week from 8 a.m. to midnight. Results are provided in a timely manner in most instances. In addition to 200 drugs covered by general screening, the tests carried out by the laboratory cover the following classes of products: street drugs, metals, pesticides (organochlorides, organophosphorous, pyrethroids), herbicides, solvents, alcohols, cyanide. The list of laboratory tests performed is available on the CTQ website.
| Detection and Isolation of Microorganisms from Food and Environmental Samples | Detection and Isolation of Microorganisms from Clinical Specimens | Identification and Characterization of Microorganisms (any source) | |
|---|---|---|---|
| Saskatchewan Disease Control Laboratory | Salmonella, Listeria, E. coli O157:H7 (including VTEC), Shigella, indicator organisms, Staphylococcus, Bacillus cereus, Clostridium perfringens, C. botulinum, Aeromonas hydrophila, Campylobacter, Yersinia, Vibrio | Salmonella, Listeria, E. coli O157:H7 (including VTEC), Shigella, indicator organisms, Staphylococcus, Bacillus cereus, Clostridium perfringens, C. botulinum, Aeromonas hydrophila, Campylobacter, Yersinia, Vibrio, parasites (if requested), viruses Viruses: nucleic acid amplification test (NAAT) for norovirus, and electron microscopy for other viruses, like rotavirus |
All foodborne/enteric bacteria: identification, speciation, serotyping, PFGE (Salmonella, E. coli, Shigella), antimicrobial resistance testing, PCR (E. coli O157:H7 VTEC) |
| Detection and Isolation of Microorganisms from Food and Environmental Samples | Detection and Isolation of Microorganisms from Clinical Specimens | Identification and Characterization of Microorganisms (any source) | |
|---|---|---|---|
| Provincial Laboratory for Public Health (ProvLab) | Food poisoning organisms: Salmonella, E .coli O157:H7, Shigella, Campylobacter, Yersinia, Aeromonas, Vibrio Indicator organisms: Staphylococcus aureus, Bacillus cereus, Clostridium perfringens Qualitative: aerobic plate count (APC) |
Stool specimens: Salmonella, E. coli O157:H7, verotoxigenic E.coli (VTEC) as requested, Shigella, Campylobacter, Yersinia, Aeromonas, Vibrio, Staphylococcus aureus, Bacillus cereus, Plesiomonas, parasites Virus: PCR (norovirus, rotavirus, astrovirus, sapovirus, enteric adenovirus) Electron microscopy (if required): Cryptosporidium, Giardia (fecal smear) |
PFGE (E. coli, Listeria, Shigella, Salmonella) Serotyping (E. coli, Shigella, Salmonella) Virus: norovirus sequencing |
| Water Parasites: Cryptosporidium and Giardia (USEPA Method 1623 and molecular speciation/ genotyping) Indicators (multiple methods): E. coli, Pseudomonas aeruginosa, fecal coliforms, total coliforms, heterotrophic plate count (HPC) |
|||
| Agri-Food Laboratories Section, Alberta Agriculture and Forestry | For full scope of accreditation, including chemical analyses of antibiotics and metals in food, visit: Accreditation Status of Agri-Food Laboratories. FM-0432 Milk and Milk Products - Determination of Alkaline Phosphatase Activity - Part 1: Fluormetric Method for Standards Council of Canada 1 Milk and Milk Based Drinks (ISO 11816-1) – Modified for Cream MFHPB-10 Isolation of Escherichia coli O157:H7/NM from foods and environmental surface samples MFHPB-18 Determination of the Aerobic Colony Count in Foods MFHPB-19 Enumeration of Coliforms, Faecal Coliforms and of E. coli in Foods using the MPN Method MFHPB-20 Isolation and Identification of Salmonella from Food and Environmental Samples MFHPB-21 Enumeration of Staphylococcus aureus in Food MFHPB-30 Isolation of Listeria monocytogenes and other Listeria spp. from foods and environmental samples MFHPB-34 Enumeration of Escherichia coli and Coliforms in Food Products and Food Ingredients using 3MTM PetrifilmTM E.coli Count Plates MFLP-15 The Detection of Listeria Species from Environmental Surfaces using the Dupont Qualicon BAX® System Method and Direct Plating MFLP-28 The Qualicon BAX® System Method for the Detection of Listeria monocytogenes in a Variety of Food MFLP-29 The Qualicon BAX® System Method for the Detection of Salmonella in a Variety of Food and Environmental Samples MFLP-30 Detection of Escherichia coli O157:H7 in select foods using the BAX® System E. coli O157:H7 MP MFLP-74 Enumeration of Listeria monocytogenes in Foods |
Description of the British Columbia Centre for Disease Control (BCCDC) Public Health Laboratory
The BCCDC Public Health Laboratory provides public health and reference clinical and environmental diagnostic services to all health authorities and other health agencies in British Columbia. It conducts reference testing of human specimens, helps identify clusters of cases based on microbiological characteristics and conducts testing of foods implicated in foodborne illness outbreaks for all foodborne pathogens, including botulism.
| Detection and Isolation of Microorganisms from Food and Environmental Samples | Detection and Isolation of Microorganisms from Clinical Specimens | Identification and Characterization of Microorganisms (any source) | |
|---|---|---|---|
| British Columbia Centre for Disease Control (BCCDC) Public Health Laboratory | Food poisoning organisms: Bacillus cereus Staphylococcus, Salmonella, Shigella, Clostridium Perfringens, Campylobacter, E. coli 0157, Vibrio Microbial Indicators: Staphylococcus Aureus, E.coli, fecal coliforms, aerobic plate counts (APC), total coliforms Drinking Water Organisms: Salmonella, Shigella, E. coli 0157, Listeria, Vibrio, Crytosporidium, Giardia, P. aeruginosa Microbial Indicators: total coliforms, E. coli, heterotrophic plate counts (HPC) |
Bacteria: Bacillus cereus, Staphylococcus Aureus, Salmonella, Shigella, Clostridium perfringens, Campylobacter, E. coli O157, E. coli non-0157, Listeria, C. botulinum Parasites: Cryptosporidium, Cyclospora, Giardia, Toxoplasma, Trichinella, Acanthaemeba, helminthes, E. histolytica Viruses: adenovirus, norovirus, sapovirus, rotavirus, astrovirus |
Bacteria: PFGE, multilocus sequencing typing (MLST) serotyping, detection of C. botulinum toxins, Shiga-toxin detection Parasites: PCR |
| Detection and Isolation of Microorganisms from Food and Environmental Samples |
Detection and Isolation of Microorganisms from Clinical Specimens |
Identification and Characterization of Microorganisms (any source) |
|
|---|---|---|---|
| Community and hospital laboratories | Stool: Salmonella, Shigella, Campylobacter, E.coli O157 and Yersinia Sterile sites: all bacterial pathogens including Listeria monocytogenes |
||
| Public Health Ontario Laboratories | Microbial indicators: Aerobic plate count, total coliform, E. coli, Total gram negative Food poisoning organisms: Bacillus cereus, Campylobacter jejuni, Clostridium perfringens, E.coli O157:H7, Listeria monocytogenes, Salmonella, Shigella, Staphylococcus aureus, Vibrio, Yersinia enterocolitica |
Bacteria: Campylobacter, Clostridium perfringens, Salmonella, Shigella, Shiga toxin producing E.coli (including E.coli O157), Yersinia enterocolitica Parasites: Cryptosporidium, Cyclospora, Giardia and other stool parasites |
Bacteria: Serotyping (Salmonella, Shigella, Shiga toxin producing E.coli including E.coli O157), PFGE (Listeria monocytogenes, Salmonella, Shigella, Shiga toxin producing E.coli including E.coli O157) |
| Detection and Isolation of Microorganisms from Food and Environmental Samples |
Detection and Isolation of Microorganisms from Clinical Specimens |
Identification and Characterization of Microorganisms (any source) |
|
|---|---|---|---|
| Cadham Provincial Laboratory | None (not done at the Provincial Laboratory) | Virus: Culture [Adenovirus, Enterovirus Electron Microscopy [Adeno, Rotavirus, Small Round Virus] Salmonella, Shigella, E. coli O157 VT+, E. coli non-O157 VT+, Staphylococcus aureus, Bacillus cereus, Campylobacter, Yersinia, Pseudomonas aeruginosa, Clostridium perfringens, Listeria, Aeromonas, Pleisomonas, Vibrio |
Virus:EIA for Enteric Adenovirus 40/41 VT detection/identification E. coli O157; VT detection E. coli non-O157; Biochemical identification of all foodborne/enteric bacteria; PFGE (Salmonella, E. coli O:157) |
Annex 10
Communicating with the public and those at greater risk: Tactics and evaluation
Communications tactics
During a foodborne illness outbreak, there will be a need to provide information and regular updates to the media, public, and other stakeholders, including those at greater risk. There will also be a need for communication among the investigative partners to ensure consistency of messaging and to draft and share messages in a coordinated manner.
The lead organization responsible for public communications, in consultation with the OICC partners and the Outbreak Communications Team (OCT), will assess the triggers for public communications (severity, scope, cause, risk perception) to determine the appropriate activities, content, and timing, for communicating about the outbreak. The OCT will make a recommendation for public communications to the OICC in relation to tactics and timing.
Communications activities could include the following:
Traditional media
Determine the appropriate media strategy (proactive or reactive), tactics (media lines, public health notice, web update, technical briefing, press release, news conference, etc.), timing, spokespersons, initial messages, and media inquiry coordination. A key media relations contact will need to be identified in each partner organization. Each organization will endeavour to have foodborne illness outbreak spokespersons who have received media relations training.
Web
Determine the appropriate web strategy, including what information is required, how it will be presented, how it will be coordinated on the websites of each involved organization, how it will be promoted, and how frequently it will be updated.
Social media
Determine the appropriate social media strategy and platforms (Twitter, Facebook, YouTube, Pinterest), including how social media messages will be promoted and coordinated among partner organizations, the frequency and timing of messages, and how messages will be monitored and responded to.
Toll-free telephone service
Determine the level of need for toll-free telephone services. The OCT lead, in consultation with the OCT members, will develop information for responding to telephone inquiries and determine if an outbreak-specific, dedicated telephone service is needed.
Communications to at-risk populations
Determine whether communications activities that target stakeholders who are at greater risk from the outbreak (due to age, gender, cultural background, or other variables) are warranted. If such a need exists, the OCT, in consultation with the OICC partners, will develop and implement communications plans and products that are specifically tailored for those stakeholders.
Key messages
Key messages and other public communications must be consistent, complementary, and developed in a timely fashion. The OCT, in consultation with the OICC partners, will coordinate the development of key messages and other content, using existing message templates whenever possible. Food safety measures and illness prevention and control measures that can be taken should be described.
The following standard messages may be used to speed the response within, for example, the first news cycle. They should not necessarily replace documents already in use for more common outbreak occurrences.
| Situation | Key Message |
|---|---|
| Human illness potentially linked to food (food source not yet confirmed) | (Statement of the current situation): There have been (number) of (type of pathogen, if known) illnesses reported in (city/P/T). (Name of lead organization – PHAC or affected P/T) is working closely with its (provincial/territorial, municipal, federal and/or international counterparts) to identify the source of the infection. At this time, the risk is (insert level of risk – low/medium/high) (Name of partner(s)) is/are investigating a number of possible sources. The necessary action will be taken to protect Canadian consumers. More information will be provided as soon as it becomes available. Some infections can spread by hand-to-hand contact with an infected person or even from surfaces he or she may have touched. Frequent hand-washing with warm water and soap will help to reduce the possibility of spreading the infection from person to person. |
| Identification of a foodborne hazard (illnesses not yet confirmed) |
(Name of lead organization – CFIA or affected P/T) is currently investigating the presence of (name of hazard, if known) in (name of food product (s)). No illnesses have been confirmed at this time (give advice on how to properly handle and cook the food in question, if it will minimize the risk). (Name of partner) is investigating in cooperation with (other federal, provincial, territorial, or municipal) counterparts. More information will be provided as soon as it becomes available. |
Communications evaluation
Formal communications evaluation should take place during and after high-profile or complex outbreaks that involve multiple communications activities. When outbreaks are simpler in scope, communications evaluation tasks may be more informal but should aim to address any misconceptions or gaps in understanding as a result of an outbreak.
During an outbreak:
Ongoing evaluation of communications plans and the activities that flow from them will be conducted by the OCT lead, in consultation with OCT members and OICC partners. Evaluation tactics will depend on the activities that are used.
Evaluation tools could include:
- media monitoring, including the volume and tone of media inquiries and media coverage;
- social media monitoring across various platforms;
- web analytics, including visitor analysis of websites;
- tracking of public inquiries via toll-free phone lines and webmail;
- informal stakeholder consultations on communications activities;
- informal or formal public opinion research in the form of online polls, omnibus surveys, or focus groups; and
- soliciting input from audiences for communications activities targeted to at-risk populations.
After an outbreak:
The post-outbreak evaluation of communications plans and the activities that flowed from them will be led by the OCT and communicated back to the OICC/be included in the OICC debrief. The evaluation tools listed above may be appropriate, as well as more involved activities that could include:
- quantitative and qualitative media analysis;
- public opinion research and analysis;
- social media research and analysis;
- behavioural research; and
- consulting audiences that received communications products targeted to at-risk populations.
Annex 11
| Steps/Issues | Comments | Action | Long-term Deliverable |
|---|---|---|---|
| Initial stage of the outbreak
Outbreak detection and surveillance systems (e.g., notification through NESP, PulseNet) Verifying the diagnosis through laboratory analysis (serotyping and PFGE); clinical information Case definitions (define and identify cases) Notification of partners (P/Ts, CDC, use of Public Health Alerts, senior management, Duty Officers) |
|||
| Outbreak Investigation Coordinating Committee
Initial Assessment Were partners given enough notice for the initial assessment call? Activation Decision-making and building consensus around activation of OICC? Were the roles and responsibilities of the FIORP Duty Officers understood? OICC Calls Were calls held frequently enough? Were the agenda and structure of the calls helpful? Were the calls well-managed? Were the right participants on the call? |
|||
| Epidemiological and Laboratory Investigations
Were the epi summaries clear, helpful, and distributed in a timely manner? Was the line list easy to understand and complete? Is there a need for alternative methods of information sharing (e.g., web-based line list; databases) |
|||
| Food safety investigation traceback data, risk assessments, recalls, plant investigation, food/environmental sampling)
Was the right information shared? Was it clear? Timely?
|
|||
| Hypothesis generation/evaluation
Develop hypotheses (interviews, interaction with local and P/T partners) Evaluate hypotheses (CDC collaboration, review of hypothesis generating interviews) Refine hypotheses and implement additional studies (analytic study methodology, preparation, questionnaire development, implementation and interviewing, analysis) |
|||
| Communications
Within OICC: Were email updates acceptable and frequent enough? Communications between OICC/FIORP Duty Officers and senior management Public communications Decision-making around public communications Internal documentation |
|||
| Public Health and Food Safety Control Measures (what was done, implementation of control and prevention measures) | |||
| Post-outbreak
(declaring the outbreak over, deactivating the OICC, debrief session, final report, Outbreak Summaries) Decision-making around declaring the outbreak over and deactivating the OICC How effective was this debrief session? Was it held in a timely manner following the outbreak? Was a final outbreak report prepared and distributed in a timely manner? (Epi report format, distribution, translation etc.) Has the outbreak been posted to Outbreak Summaries? |
|||
| Other issues? |
Annex 12
F/P/T Detailed Roles and Responsibilities
Newfoundland and Labrador
The Newfound and Labrador (NL) Department of Health and Community Services, Regional Health Authorities and Service NL (formerly Department of Government Services) are involved in the investigation of foodborne illness outbreaks. Investigation and management of foodborne illness outbreaks occur as outlined in the NL Disease Control Manual and in accordance with the Memorandum of Understanding between Health and Government Services. The Department of Natural Resources, Animal Health Division, is the provincial lead for animal health, raw milk quality, and meat inspections.
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type/Scope |
|---|---|---|---|---|---|
| Department of Health and Community Services | Health and Community Services (Public Health Division) and Regional Health Authorities | Food and Drug Act
Communicable Diseases Act |
Food Premises Regulations Memorandum of Understanding (MOU) between Health and Government Services Communicable Disease Manual |
Food safety program/policy lead
Disease surveillance Outbreak investigation lead Epidemiology |
All (e.g. food, retail and food service establishments), except those that are federally registered and the regulatory responsibility of the Canadian Food Inspection Agency (CFIA) |
| Service NL | Government Service Centre | Food and Drug Act
Communicable Diseases Act |
Food Premises Regulations MOU between Health and Government Services Communicable Disease Manual |
Inspections
Investigation Clinical specimens, food samples, and environmental samples |
All (e.g. food, retail and food service establishments), except those that are federally registered and the regulatory responsibility of the CFIA |
| Department of Natural Resources | Animal Health Division | Food and Drug Act
Meat Inspection Act |
Food Premises Regulations
Meat Inspection Regulations |
Program/policy lead
Surveillance Investigation lead |
Dairy farms Abattoirs |
| Service NL | Government Service Centre | Food and Drug Act
Meat Inspection Act |
Food Premises Regulations
Meat Inspection Regulations |
Inspections
Raw milk sampling Meat inspection |
Dairy farms Abattoirs |
Prince Edward Island
Summary: The Prince Edward Island (PEI) Department of Health and Wellness is involved in the investigation of foodborne illness outbreaks. Outbreak investigations would be coordinated through the office of the Chief Public Health Officer. Provincial government departments within the jurisdiction as well as the CFIA could be involved.
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
| PEI Department of Health and Wellness | Chief Public Health Office | Public Health Act | Food Premises Regulations
Slaughterhouse Regulations Guidelines for Poultry Killing Facilities Cottage Industry Guidelines |
Food safety investigations | All types
Slaughterhouses Poultry killing facilities Manufacturing |
| Department of Agriculture and Fisheries | Fish Inspection Act Fisheries Act |
Add GF2 Funding/ Traceability Fish Inspection Act, Regulations |
Fish plants |
Nova Scotia
Summary: In Nova Scotia, the role of investigating and controlling foodborne illness outbreaks is the responsibility of both the Environmental Health and Food Safety Programs, Nova Scotia Environment (NSE), the Office of the Chief Medical Officer of Health in the Department of Health and Wellness (OCMOH) and Public Health in the Nova Scotia Health Authority. The usual first point of contact is with Health and Wellness. Investigation and management of an outbreak follows the Outbreak Response Plan (August 2015) chapter as contained in the Nova Scotia Communicable Disease Control Manual. The Environmental Health and Food Safety Programs, NSE contributes through its food safety investigation as well as enforcement and regulatory control. Public Health, Nova Scotia Health Authority contributes through case investigation and management.
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
| NSE | Food Safety Section | Health Protection Act Meat Inspection Act |
Food Safety Regulations
Meat Inspection Regulations MOU between Agriculture and Fisheries and Health |
Food safety investigations | All establishment types |
| Department of Health and Wellness | OCMOH with Public Health, Nova Scotia Health Authority | Health Protection Act | Outbreak Response Plan
Notifiable Diseases Reporting System in Nova Scotia |
Public health surveillance, case investigation, management (public health measures), outbreak management and publicly funded immunization as related to food and waterborne outbreak response, Medical Officer of Health orders |
New Brunswick
Summary: The Department of Health maintains surveillance and investigation and control programs for foodborne illness in the province. The usual first point of contact is one of our Regional Public Health Offices, where reports are received from the public, laboratories, and health care providers. The department has developed standard disease guidelines in the New Brunswick Reportable Diseases and Events Guide (RDEG). The RDEG articulates the roles and responsibilities of the Outbreak Response Team, which may include federal members and other provincial departments.
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
| Department of Health | Public Health | Public Health Act | Reg. 2009-136
(Reporting Disease) Reg. 2009-138 (Food Premises) Reg. 2009-139 (Dairy Plant and Transportation of Milk) Reg. 2009-140 (Abattoir) Reg. 2009-141 (Health Regions) New Brunswick Reportable Diseases and Events Guide (RDEG) Other policy directives as required National guidelines where applicable and available |
Notifiable diseases and reportable events listing
Reporting requirement Investigation and control authority |
Communicable diseases investigation is not limited to a type of establishment but to the outbreak. Should a suspected food source be from an establishment under regulatory jurisdiction of another agency, that agency would be involved (i.e. CFIA) |
Quebec
Summary: In Quebec, two government ministries are responsible for the biofood component of public health protection (other ministries, such as the Ministère du Développement durable, de l'Environnement et des Parcs (MDDEP), are also responsible for protecting the health of the public): the Ministère de la Santé et des Services sociaux (MSSS) and the Ministère de l'Agriculture, des Pêcheries et de l'Alimentation (MAPAQ). These organizations work together to resolve foodborne illness incidents by conducting food safety and epidemiological investigations. Provincially, the coordination of outbreak responses involves two separate levels: human cases are investigated by MSSS (supraregional and national outbreaks) or the public health branches (Directions de la Santé Publique (DSPs)) (regional outbreaks) and cases involving bio-food establishments are investigated by MAPAQ.
In 1996, MAPAQ, MSSS, the DSPs, and the Institut national de santé publique du Québec (INSPQ) signed a cooperation and personal information communication agreement. This agreement, which was updated in 2007, enables stakeholders to share information and to carry out more extensive investigations in their area of expertise, be it affected individuals (public health) or food and food establishments (MAPAQ). The agreement also includes the Laboratoire de santé publique du Québec, which detects and identifies pathogens from affected persons and conducts fine characterization (pulsed-field gel electrophoresis (PFGE)) of microorganisms on both the human health side and the dietary-environmental side.
MAPAQ has also established an agreement with the CFIA for all products from outside Quebec and all products made in Quebec but destined for international trade. The goal of this agreement is to enhance cooperation in the field of food inspection, to use resources more efficiently, and to facilitate domestic and international trade by providing harmonized inspection programs and services.
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
| Agriculture, Pêcheries et Alimentation (MAPAQ) |
Direction générale de la santé animale et de l'inspection des aliments (DGSAIA) | Food Products Act R.S.Q., P-29 An Act to Regularize and Provide for the Development of Local Slaughterhouses R.S.Q., chapter R-19.1 Marine Products Processing Act (T11.01) |
Regulation respecting food (c. P-29, r.1):
-C.1 General provisions c. P-29, r.1.1 Regulation respecting bottled water c.T-11.01, r.1- Regulation respecting minimum standards for processing marine products c.T-11.01, r.2- Regulation respecting permits for acquirers of marine products CFIA-MAPAQ memoranda of understanding concerning inspection activities Cooperation and information-sharing agreement concerning the prevention, surveillance and control of food poisoning and foodborne illness between MAPAQ, MSSS, regional DSPs, and INSPQ Regulation to designate contagious or parasitic diseases, infectious agents and syndromes (CQRL, c. P-42, r.4.2) |
Issuance of operator permits for slaughterhouses, food processing plants (meat, milk and seafood products), restaurants, and retail outlets Issuance of permits to salvagers and dismembering plants Inspection of all types of food establishments according to risk-based inspection Ongoing inspection of class A slaughterhouses. Investigation of food-related complaints Food recalls and CFIA recall effectiveness audits Application of CFIA programs for tertiary sector establishments Inspection of premises and vehicles used to hold/transport marine products Provincial coordination of food poisoning incidents Provincial coordination of risk assessments Food testing (microbiological, chemical, physical) Food safety investigations Establishment closings Permits suspension Annual test programming Surveillance of contagious or parasitic diseases, infectious agents and syndromes Health measures order Emergency measures |
Retail food outlets, restaurants, food distributors Egg producers, egg classification and processing stations Slaughterhouses and meat processing plants Producers, salvagers and dismembering plants Seafood and freshwater product plants and canneries Farms and dairy plants, milk transporters and distributors, dairy product substitute producers and wholesalers Other food producing plants Water bottling plants and bottled water distributors Fresh produce growers and packagers, retail outlets, and green grocers Local slaughterhouses, meat and meat product processing plants for retail sale Package butcher shops (meat) All types of supraregional, national, and international food poisoning outbreaks |
| Santé et Services sociaux (MSSS) | Bureau de surveillance et de vigie des éclosions | Public Health Act | Legislation and regulations List of notifiable diseases Cooperation and information-sharing agreement concerning the prevention, surveillance, and control of food poisoning and foodborne illness between MAPAQ, MSSS, regional DSPs, and INSPQ |
Provincial infectious disease surveillance Coordination of supraregional outbreaks Epidemiological investigations |
All types of supraregional, national, and international food poisoning outbreaks |
| Directions régionales de santé publique (18) | Public Health Act | Legislation and regulations Cooperation and information-sharing agreement concerning the prevention, surveillance and control of food poisoning and foodborne illness between MAPAQ, MSSS, regional DSPs, and INSPQ |
Processing of regional notifiable disease reports Regional reception and reporting Regional infectious disease surveillance Epidemiological investigations Coordination of regional outbreak responses |
All types of regional food poisoning outbreaks | |
| INSPQ | Laboratoire de santé publique du Québec | An Act respecting Institut national de santé publique du Québec Public Health Act |
Cooperation and information-sharing agreement concerning the prevention, surveillance and control of food poisoning and foodborne illness between MAPAQ, MSSS, regional DSPs, and INSPQ | Identification, confirmation, and fine characterization of pathogens (PFGE) | All types of regional, supraregional, national and international food poisoning outbreaks
Biological sampling Environmental bacteria strains from MAPAQ Pathogens of all types |
Ontario
Summary: The Ontario Ministry of Health and Long Term Care (MOHLTC), local boards of health and agency partners are involved in the investigation and control of foodborne illness outbreaks. The MOHLTC is the lead and coordinating agency for foodborne illness outbreaks in Ontario. It provides advice and policy direction to the boards of health and administers legislation for the organization and delivery of public health programs and services and the prevention of the spread of disease.
The 36 boards of health, located across the province, are responsible for the inspection of non-federally registered food processing plants, food retail establishments (including restaurants, nursing homes, and hospitals) and for responding to food-related complaints and investigating foodborne disease outbreaks. Boards of health also have broad powers to take action (i.e. issue tickets under the Provincial Offences Act, lay charges, condemn food, and order an establishment closed under the Health Protection and Promotion Act) to protect public health.
The Ontario Agency for Health Protection and Promotion (OAHPP) is mandated to provide scientific and technical advice to those working to promote and protect the health of Ontarians.
The Ontario Ministry of Agriculture, Food and Rural Affairs (OMAFRA) contributes to the prevention, investigation, and control of foodborne illness outbreaks through their regulatory administration, compliance, and enforcement activities. OMAFRA is responsible for legislation dealing with a variety of matters, such as conducting inspection, compliance, and investigation services.
Notes:
1. Statutes and regulations may be viewed electronically on the E-Laws website
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
| MOHLTC | MOHLTC and local boards of health | Health Protection and Promotion Act (HPPA) | Reg. 562 (Food Premises)
Reg. 554 (Camps in Unorganized Territory) Reg. 568 (Recreational Camps) Ontario Public Health Standards (2008) and Protocols, (e.g. Food Safety Protocol, Infectious Diseases Protocol) Guidance Document For Inspecting Meat Plants and Food Premises) |
MOHLTC coordinates response to foodborne disease outbreaks. Boards of health provide inspection and enforcement services for retail food premises. Food recall activities |
Food premises, including restaurants, daycares, butcher shops and health care facilities Camps and camps used for recreational activities |
| The Ontario Agency for Health Protection and Promotion (OAHPP) | Ontario Agency for Health Protection and Promotion Act | Operates and maintains laboratory centres and provides laboratory services Provides scientific and technical advice and operational support to any person or entity in an emergency or outbreak situation that has health implications, as directed by the Chief Medical Officer of Health of Ontario |
|||
| OMAFRA | Food Inspection Branch Animal Health and Welfare Branch (with MNR as OMAFRA enforcement partner) |
Food Safety and Quality Act | Reg. 31/05
(Meat) Reg. 222/05 (General) Reg. 223/09 (Fees) Reg. 266/09 (Livestock and Poultry Grades and Sales) Reg. 105/09 (Disposal of Deadstock) |
Inspection and Enforcement | Meat plants, including abattoirs and free-standing meat plants Premises that grade poultry, lamb and mutton, beef and veal carcasses Any non-farm premise on which there is a dead animal of the species to which the Act applies, and the premises of collectors, |
| Food Inspection Branch | Fish Inspection Act | Reg. 456 (Quality Control) | Interim audit program and environmental swabbing | Non-federally registered fish processors | |
| Food Inspection Branch | Food Safety and Quality Act | Reg. 119/11 Produce, Honey and Maple Products | Testing, advisory, and compliance | Premises of producers, packers, distributors, and retailers and any premise where regulated activity is carried out |
|
| Animal Health and Welfare Branch | Livestock and Livestock Products Act |
Reg. 724 (Eggs) Reg. 726 (Processed Eggs) Reg. 318/99 (Livestock and Livestock Products) |
Premises where eggs are graded or processed, any place handling non-ambulatory livestock, abattoirs |
||
| Animal Health and Welfare Branch | Livestock Community Sales Act |
Reg. 729 (General) |
Inspection and Enforcement | Any premise where community sales of livestock are conducted |
|
| Food Inspection Branch and Dairy Farmers of Ontario | Milk Act | Reg. 753 (Grades, Standards, Designations, Classes, Packing and Marking) Reg. 761(Milk and Milk Products) |
Inspection and Enforcement | Any premise where milk or dairy products are produced, manufactured, processed, graded or sold |
|
| Ministry of the Environment (MOE) and OMAFRA | MOE Operations Division with OMAFRA Environmental Management Branch |
Nutrient Management Act | Reg. 106/09 (Disposal of Dead Farm Animals) | OMAFRA(Advisory)
MOE (Inspection and Enforcement) |
Agricultural operations that have dead animals to which the Act applies |
Manitoba
Summary: Manitoba Health, Seniors and Active Living and Manitoba Agriculture may be involved in foodborne illness investigations pursuant to The Public Health Act. Additionally, public health nurses with Regional Health Authorities also play a significant role in interviewing and case follow-up. Cadham Provincial Laboratory conducts testing on human isolates and an accredited private laboratory conducts food sample testing, funded by Manitoba Health Seniors and Active Living. Manitoba Agriculture provides inspection at provincially regulated food processors, including provincial abattoirs.
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
| Manitoba Health, Seniors and Active Living | Public Health and Primary Health Care Branch, and Office of the Chief Provincial Public Health Officer
Cadham Provincial Laboratory |
The Public Health Act | Reg. 339/88R
(Food and Food Handling Establishments) Reg. 37/2009 (Reporting of Diseases and Conditions) Reg. 26/2009 (Disease Control Regulation) Reg. 29/2009 (Health Hazards Regulation) Communicable Disease Management Protocols, including Enteric Illness Protocol Manuals City of Winnipeg Food Service Bylaw (City of Winnipeg only) |
Policy and legislation development; laboratory testing; food safety inspections; foodborne illness and complaint investigations; reportable disease surveillance; epidemiological analysis; food recalls (within province) Measures to prevent further human cases from developing, which may include public communication |
All food establishments, food sources Coordination of foodborne illness investigation Medical officers of health responsible for foodborne illness investigation Public health inspectors responsible for all retail food establishments |
| Regional Health Authorities | Public Health Nurses
Regional epidemiologists |
The Public Health Act | Communicable Disease Management Protocols, including Enteric Illness Protocol manuals | Public health nurses with Regional Health Authorities play a significant role in patient interviewing and case follow-up | Initial interviews and case follow-up of patients for all reportable diseases Medical officers of health responsible for foodborne illness investigation at the regional level |
Manitoba Agriculture |
CVO / Food Safety Branch | Dairy Act (Manitoba) The Public Health Act |
Reg. 137/2015 (Dairy Regulation, Amendment) Reg. 138/2015 (Dairy Farms Regulation) Reg. 339/88R (Food and Food Handling Establishments Regulation) |
Food safety investigations in food processing establishments and provincial abattoirs, in cooperation with other regulatory agencies | Health officer responsible for inspection of food processing establishments, provincial abattoirs, provincial dairy processing plants and dairy farms |
| The Animal Diseases Act | Reg. 59/2007 (Reportable Diseases) | Surveillance, outbreak investigation concerning animal diseases | The definition of disease includes conditions that may cause products derived from a diseased animal to be unsafe or unfit for use or consumption Program focus on pre-slaughter establishments |
Saskatchewan
Summary: Regional health authorities, Saskatchewan Ministry of Health, and partner agencies are responsible for investigation and mitigation of foodborne illness outbreaks. First point of contact for an outbreak within a geographic area is the regional health authority. The Saskatchewan Ministry of Health will assist in coordinating a multi-jurisdictional outbreak. Other agencies, including the Saskatchewan Ministry of Agriculture, CFIA, and Health Canada contribute to investigations through their food safety inspection, investigation, and food recall activities. As well, PHAC does provide epidemiological support when requested.
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
| Ministry of Health | Regional Health Authorities / Sask. Health | The Public Health Act, 1994 | Disease Control Regulations
Food Safety Regulations Milk Pasteurization Regulations Communicable Disease Control Manual Agreements to be developed with CFIA, Saskatchewan Ministry of Environment, Saskatchewan Ministry of Agriculture, and HC to address local roles and responsibilities for food safety and inspections of food processing facilities |
Food safety investigations Epidemiological investigations |
All facilities for which regional health authorities are the lead jurisdiction |
Alberta
Summary: A Foodborne Illness and Risk Investigation Protocol (FIRIP) is maintained by the Canada-Alberta Partners in Food Safety (CAPiFS), which includes representatives of CFIA, HC, Alberta Health (AH), Alberta Health Services (AHS) (including the Alberta Provincial Laboratory for Public Health) and Alberta Agriculture and Forestry (AAF). AH, via AHS, coordinates the investigation and control of foodborne and waterborne illness outbreaks. The usual first point of contact is the Environmental Public Health Program of AHS. AHW or other ministries/agencies, such as ARD and CFIA may be involved, depending on the nature of the outbreak and the suspected food source.
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
| Alberta Health | Alberta Health Services | The Public Health Act | Communicable Diseases Regulation Food Regulation Alberta Foodborne Illness and Risk Investigation Protocol |
Food safety investigations Foodborne illness investigations |
All food processing, services and retail establishments, unless CFIA-registered or inspected by ARD |
| Alberta Agriculture and Forestry | Regulatory Services Division
Food Safety and Animal Health Division |
Meat Inspection Act Dairy Industry Act Animal Health Act |
Meat Inspection Regulation
Dairy Industry Regulation Purchase and Sale of Eggs and Processed Egg Regulation Honey Grading Regulation |
Investigations related to abattoirs, mobile butchers, and provincially licensed dairies, and animal diseases and toxic adulterant exposure
Support of on-farm food safety programs Reportable diseases (including zoonotics and toxins that could affect food safety) Hazard Analysis Critical Control Point System (HACCP) and Good Manufacturing Practices (GMP) training in processing facilities Poultry health program Laboratory support for food processors and producers; food safety surveillance; food safety consultation for all producers and processors |
Mobile butchers Abattoirs Provincially licensed dairies |
British Columbia
Summary: The Ministry of Health and partner agencies are responsible for the investigation and mitigation of foodborne illness outbreaks. The first contact for outbreaks within a geographic region is the Regional Health Authority. Coordination of multi-jurisdictional outbreaks is usually the responsibility of Communicable Disease Prevention and Control Services, BC Centre for Disease Control (BCCDC). Reference diagnostic testing is provided by the BCCDC Public Health Laboratory (BCCDCPHL). Other agencies, including Food Protection Services of BCCDC, the Ministry of Agriculture, CFIA, and Health Canada contribute to investigations through their food safety investigation and recall activities, as well as their regulatory compliance and enforcement activities.
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
| Ministry of Health | Various branches | Public Health Act
Food Safety Act |
Food Premises Regulation Sanitary Regulations Meat Inspection Regulation (except as it relates to slaughter which is responsibility of Ministry of Agriculture) |
Outlines public health requirements for food premises that supply and serve food to the public
Sets out regulatory regime for licensing, inspecting, and responding to complaints regarding food premises Sanitary requirements for different businesses, including food establishments Outlines powers of the Medical Health Officer (MHO) and inspectors in responding to unsanitary conditions at food establishments Outlines public health requirements for all food-related businesses (farm to fork) that produce, supply, and serve food to the public Sets out regulatory regime for licensing, inspecting, and responding to complaints regarding food industry Includes legislative authority for recall of food The regulation governs the slaughter of animals for food sold in BC and provides for consistent standards in relation to licensing, inspection, and slaughter across the province |
Food premises conducting business within BC, excluding federally registered establishments Persons selling foods Food establishments conducting business within BC, excluding federally registered establishments Meat processing at slaughter establishments conducting business within BC, excluding federally registered plants |
| Provincial Health Officer | Public Health Act | Communicable Disease Regulation | Provides authority for all matters pertaining to health and disease prevention Establishes governance framework for public health and tools for preventing and removing a broad range of health hazards Sets out roles and responsibilities of the Provincial Health Officer (PHO) in relation to outbreak management and oversight of other public health professionals, as well as advisory and educational roles provided to the Minister and the BC public |
Standards for a variety of institutions, facilities, and businesses that could pose a risk to public health (e.g. processing plants, restaurants, and grocery stores) | |
| Health authorities | Public Health Act
Food Safety Act Drinking Water Protection Act |
Food Premises Regulation Drinking Water Protection Regulation Communicable Disease Regulation Meat Inspection Regulation (except as it relates to slaughter which is responsibility of Ministry of Agriculture) Sanitary Regulations BC FIORP MOU to facilitate the sharing of information for the purpose of investigating and controlling a confirmed or suspected foodborne illness in BC |
Local foodborne illness investigations, food safety investigations | All food premises, excluding federally registered establishments and those listed under Food Protection Services, BCCDC | |
| Communicable Disease Prevention and Control Services, BCCDC | NA | MOU between the Office of the Provincial Health Officer and the Ministry of Health and the BC Centre for Disease Control, Provincial Health Services Authority (PHSA) and BC FIORP MOU to facilitate the sharing of information for the purpose of investigating and controlling a confirmed or suspected foodborne illness in BC |
Epidemiological surveillance, coordination, investigation, reporting.
Coordination with PHAC and provinces for interprovincial outbreaks |
Foodborne, zoonotic infectious disease outbreaks | |
| Food Protection Services, BCCDC | Food Safety Act Milk Industry Act |
Dairy Processing Plant Regulations Milk Industry Standards Regulation MOU between the Office of the Provincial Health Officer and the Ministry of Health and the BC Centre for Disease Control, PHSA BC FIORP MOU to facilitate the sharing of information for the purpose of investigating and controlling a confirmed or |
Food Safety investigations at dairy processing plants Coordination of provincial response to recalls and multiple jurisdiction outbreaks |
Dairy processing plants and licensed dairy farms. | |
| British Columbia Public Health Laboratory, Provincial Health Services Authority | Health Act | Communicable Diseases Regulations MOU Provincial Health Officer |
Lab testing for food implicated in outbreaks Surveillance for clusters of confirmed cases Public health clinical and food testing and fingerprinting of organisms |
All types of foodborne pathogens, including botulism | |
| Ministry of Agriculture | Dairy Program, Regulatory Unit | Food Safety Act
Milk Industry Act Fish and Seafood Act Animal Health Act |
Meat Inspection Regulation Milk Industry Standards Regulation Fish and Seafood Licensing Regulation |
Licensing and inspection of slaughter establishments Establishment of standards for the production, storage and transportation of raw milk. Licensing and inspection of dairy farms, milk transporters, and milk storage equipment on farm. Prevention of antibiotic residues in milk as well as other contaminants. Setting standards for milk quality, and the application of warnings and penalties for violation of milk quality standards. Governs fish and shellfish harvested, processed, and sold in BC, the licensing of fish processing plants, and the inspection of these premises AHA provides the Chief Veterinary Officer with powers to investigate zoonotic disease in all animals including those on farms |
Slaughter Establishments Dairy Plants Seafood Processing Plants All animals in the province |
Nunavut
Summary: The Department of Health is involved in the investigation of foodborne illness outbreaks in Nunavut. Outbreak investigations would be coordinated through the office of the Chief Medical Health Officer. The first point of contact is the Health officers responsible for handling food borne illness outbreaks.
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
| Department of Health | Chief Medical Officer of Health | Public Health Act | Eating and Drinking Places Regulations
Communicable Diseases Regulations |
Outlines requirements for food premises that supply and serve food to the public. Food Safety investigations Foodborne illness investigations Public health surveillance Epidemiological investigations |
All food premises. All establishment types |
Northwest Territories
Summary: The Chief Public Health Officer, within the Department of Health and Social Services, is involved in the investigation and control of foodborne illness outbreaks in the Northwest Territories. The first point of contact is the Chief Public Health Officer and Public Health Officers responsible for foodborne illness outbreaks.
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
| Department of Health and Social Services | Chief Public Health Officer | Public Health Act | Food Establishment Safety Regulations
Disease Surveillance Regulations Reportable Disease Control Regulations |
Food safety Food safety and |
Any establishment where food intended for human consumption is manufactured, processed, prepared, packaged, stored, handled, displayed, transported, distributed, served, offered for sale, or sold Any reportable disease that can be spread through food (not necessarily specific to food establishments) |
Yukon
Summary: The Chief Medical Officer of Health, in conjunction with the Department of Health and Social Services, is involved in the investigation and control of foodborne illness outbreaks in Yukon. The usual first point of contact is the Department of Health and Social Services, represented by Yukon Communicable Disease Control and or Environmental Health Services.
| Ministry Responsible | Lead Agency | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
Department of Health and Social Services |
Yukon Communicable Disease Control | Public Health and Safety Act | Communicable Diseases Regulations | Foodborne illness investigations Public health surveillance Epidemiological investigations |
All establishment types |
| Environmental Health Services | Public Health and Safety Act | Eating or Drinking Regulations Public Health Regulations |
Food safety inspections
Sampling |
Food service /retail facilities (permanent and temporary) Care facilities Institutional facilities |
Government of Canada
Summary: The Government of Canada is involved in the investigation and control of foodborne illness outbreaks. The usual first point of contact is the Centre for Foodborne, Environmental and Zoonotic Infectious Diseases (CFEZID), within the Public Health Agency of Canada (PHAC). Health Canada (HC) Branches and Agencies may also be involved according to their respective mandates. The Canadian Food Inspection Agency (CFIA) contributes to the investigation and control of foodborne illness outbreak through its food safety investigation and recall activities, as well as its regulatory compliance and enforcement activities. The following table provides further details on the Government of Canada responsibilities for activities related to foodborne illness investigations.
| Lead Agency | Branch(es) | Legislation | Regulations, Policies, MOUs | Activity Type | Establishment Type / Scope |
|---|---|---|---|---|---|
| PHAC | PHAC | Public Health Agency of Canada Act
Department of Health Act Human Pathogens and Toxins Act Quarantine Act |
International Health Regulations Human Pathogens Importation Regulations Foodborne Illness Outbreak Response Protocol (FIORP) custodian |
Public health surveillance Epidemiological investigations |
|
| HC | Healthy Environments and Consumer Safety Branch | Department of Health Act Canada Consumer Product Safety Act |
Investigations involving consumer products | Consumer products which could pose an unreasonable danger to human health or safety | |
| HC | First Nations and Inuit Health Branch | Department of Health Act Constitution Act (s. 91(24)) |
1979 Indian Health Policy (s. 88) | Investigations/surveys involving First Nations communities on reserves south of 60 degrees parallel | All establishment types located in First Nations communities on reserves south of 60 degrees parallel |
| HC | Health Products and Food Branch | Food and Drugs Act | Food and Drug Regulations | Provision of health risk assessments to CFIA and provinces/territories upon request | All types of hazards related to food and veterinary drugs that fall under the scope of the Food and Drugs Act and its Regulations |
| HC | Pest Management Regulatory Agency | Pest Control Products Act Food and Drugs Act |
Food and Drug Regulations (Provision B.15.002(1); 0.1 ppm MRL) MOU between the CFIA and the PMRA |
Assistance to CFIA or other stakeholders upon request for food safety investigations involving exceedences of pesticide MRLs (including provision of health risk assessments) | All establishment types |
| CFIA | CFIA | Food and Drugs Act | Food and Drug Regulations (regarding food and veterinary drugs) 1999 MOU between Health Canada and the CFIA 1999 Roles and Responsibilities Framework for Federal Food Safety and Inspection Activities (HC and CFIA) |
Food safety investigations | All establishments types |
| CFIA | CFIA | Canadian Food Inspection Agency Act Canada Agricultural Products Act Consumer and Packaging Labelling Act Fish Inspection Act Health of Animals Act Meat Inspection Act |
Dairy products, Egg, Fresh Fruit and Vegetable, Honey, Maple Products, Processed Egg, and Processed Products Regulations Organics Regulations Consumer Packaging and Labelling Regulations Fish Inspection Regulations Health of Animals, Reportable Diseases Regulations Meat Inspection Regulations |
Food safety investigations | Registered food processing establishments, growers, packers, importers |
Annex 13
Standard Operating Procedure (SOP) for Directing Food Samples Collected by Provincial/Territorial/Municipal Inspectors during Epidemiological/Public Health/Food Safety Investigation to the Federal Laboratory Network
Standard Operating Procedure (SOP) for Directing Food Samples Collected by Provincial/Territorial/Municipal Inspectors during Epidemiological/Public Health/Food Safety Investigations to the Federal Laboratory Network
Background:
Following the listeriosis deli-meat outbreak in the summer of 2008, it was recognized that there was a need to address the process for tracking and directing food samples taken by provincial/territorial/municipal/public health/agriculture (P/T/M) Inspectors related to a possible food safety incident that may need to enter into the Federal Laboratory Network system. In addition, it was also noted that there was a need to improve the communication and information sharing mechanism among all partners involved in the investigation on the receipt of, analysis and reporting of test results on food samples.
Food samples collected by P/T/M Inspectors, (including those collected by public health inspectors and Agriculture Ministries) in support of an epidemiological/public health/food safety investigation are routinely submitted for analysis to the Provincial Public Health Laboratories (PPHLs). When food samples cannot be analysed within the provincial network (public health and/or agri-food), either due to the lack of specific expertise or capacity, the samples can be directed for analysis to the Federal Laboratory Network by following the procedures described in this SOP. The Area/Regional CFIA Operations designated contacts will provide the liaison between P/T/M Inspectors and the Federal Laboratory Network. This process is depicted in Appendix A.
Process
1. During an epidemiological/public health/food safety investigation, the P/T/M Inspector or other food safety professional collects a food sample for testing and completes a sample collection form with as much information as possible. Wherever possible, sufficient sample should be submitted for analysis (usually 5 x 200g) to ensure Health Canada has sufficient information to conduct a health risk assessment. An example of the sample collection form is attached in Appendix B.
2. Exception: Clostridium botulinum
When the testing requested is for Clostridium botulinum, inquiries are to be directed immediately to the Botulism Reference Service.
Botulism Reference Service: Chair (office (613-957-0902), cell (613-296-1139); Analyst (lab 613-957-0885), Health Products and Food Branch, Health Canada, Banting Research Centre, 251 Sir Frederick Banting Driveway, Ottawa, Ontario, K1A 0L2, Postal Locator 2204A2
For samples from British Columbia, contact BCCDC Lab: Medical Microbiologist on call at 604-661-7033.
3. The P/T/M Inspector determines if the food sample can be analyzed by the PPHL. If there is laboratory capacity and expertise, the P/T/M Inspector sends the sample along with the sample collection form to the appropriate PPHL laboratory for testing.
4. If it is determined that the PPHL does not have the capacity or expertise to analyze the food sample or does not know, the P/T/M Inspector contacts the appropriate Area/Regional CFIA Operations designated contact for assistance. The list of Area/Regional CFIA Operations designated contacts (i.e. Area Recall Coordinator (ARC), Regional Recall Coordinator (RRC) including back-ups) can be found in the FIORP Contact List.
The P/T/M Inspectors, when requesting the analysis of food samples by the Federal Laboratory Network, should provide the following information to the Area/Regional CFIA Operations designated contact:
- reason why the samples were collected
- number of samples collected for the analysis
- estimated time when the samples will arrive at the laboratory
- laboratory analysis required
- available details on any case investigation(s) associated with the samples
In addition, the sample collection form (Appendix B) should be as complete as possible.
5. After receiving the request from the P/T/M Inspector, the Area/Regional CFIA Operations designated contact should contact the CFIA's Food Safety Science Directorate (FSSD) to determine the closest lab with the required expertise and capacity. A discussion/teleconference should be held with the P/T/M Inspector, the Area/Regional CFIA Operations designated contact and FSSD to determine the appropriate CFIA laboratory with the required expertise, and FSSD will confirm that the identified laboratory is ready to accept the samples. If the expertise does not reside in the CFIA or if the capacity of the CFIA has been exceeded, the FSSD will contact its federal partners, HC and/or PHAC, for assistance and will confirm the selected laboratory with the P/T/M Inspector and the Area/Regional CFIA Operations designated contact. Contact information for FSSD is provided in Appendix C.
6. The P/T/M Inspector will forward the sample to the Area/Regional CFIA Operations designated contact packaged in a manner that is ready to ship to the federal laboratory (i.e. properly packaged with ice packs).
7. The Area/Regional CFIA Operations designated contact should ensure that all information, including Submitter Lab Numbers, accompanying the sample is entered into the CFIA Issue Management System (IMS) database, (i.e. all details, on the investigation associated with the samples(Appendix B: Sample Collection Form, provided by the P/T/M Inspector)).
8. The Area/Regional CFIA Operations designated contact ensures that a LSTS submission is created and submitted under the appropriate sampling plan code (i.e. MX200, OFSR206). No sample number is required.
9. The Area/Regional CFIA Operations designated contact sends the samples along with the completed sample collection form to the appropriate federal laboratory. Under most circumstances, human resources, shipping material and courier expenses related to this activity are to be covered by Operations Branch. Any questions or concerns related to expenses associated with this activity should be directed to your supervisor.
10. Upon receipt of the sample the CFIA federal laboratory shall confirm receipt with the Area/Regional CFIA Operations designated contact.
11. Lab results from the federal laboratories will be communicated to the Area Recall Coordinator and the Area/Regional CFIA Operations designated contact who will then re-direct the results to the P/T/M Inspector.
12. The investigation will continue as per routine procedures or as per the Foodborne Illness Outbreak Response Protocol (FIORP) in the case of a multi-jurisdictional foodborne illness outbreak. Appendix A - Process Diagram for Directing Food Samples Collected by Provincial/Territorial/Municipal Inspectors during Epidemiological /Public Health/Food Safety Investigations to the Federal Laboratory Network
Appendix A - Process Diagram for Directing Food Samples Collected by Provincial/Territorial/Municipal Inspectors during Epidemiological /Public Health/Food Safety Investigations to the Federal Laboratory Network
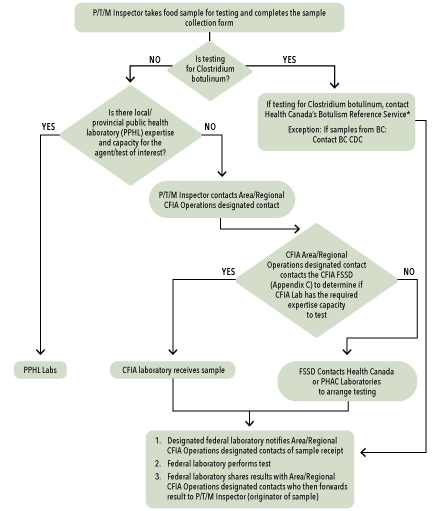
Figure 3: Process Diagram for Directing Food Samples Collected by Provincial/Territorial/ Municipal Inspectors during Epidemiological/Public Health/Food Safety Investigations to the Federal Laboratory Network
Appendix A represents the general process for directing food samples collected by Provincial/Territorial/Municipal (P/T/M) Inspectors during an epidemiological/public health/food safety investigation to the Federal Laboratory Network.
The procedure would typically begin with a P/T/M inspector taking a food sample, related to a possible food safety incident, for testing and then completing a sample collection form.
The first question that needs to be addressed is whether this is a sample to be tested for Clostridium botulinum. If Yes, then inquiries are to be directed immediately to Health Canada's Botulism Reference Service. The Botulism Reference Service notifies partners of receipt of sample (HC, PHAC, CFIA, P/T/M, etc.). The laboratory performs the test and shares the results with P/T/M inspector (originator of the sample), PHAC, HC and CFIA.
There is an exception: if the sample to be tested for Clostridium botulinum is from British Columbia, the P/T/M inspector would contact the BCCDC, Environmental Microbiology Lab. The BCCDC laboratory notifies partners of receipt of sample (HC, PHAC, CFIA, P/T/M, etc.), performs the test and shares the results with P/T/M inspector (originator of the sample), PHAC, HC and CFIA.
If the test is not for Clostridium botulinum, then the P/T/M inspector determines if there is local/provincial public health laboratory (PPHL) expertise and capacity for the agent/test of interest. If Yes, then the sample is sent to the PPHL Labs. If No, then the P/T/M inspector needs to contact Area/Regional CFIA Operations designated contact. This CFIA representation will contact the CFIA Food Safety Science Directorate (FSSD) to determine if CFIA Lab has the required expertise and capacity to test the sample (see Appendix C). If Yes, then the sample is sent to CFIA laboratory. If the CFIA Lab is not able to test the sample then the FSSD contacts Health Canada or PHAC laboratories to arrange testing. In both cases, the federal (CFIA, Health Canada, or PHAC) laboratory notifies the Area/Regional CFIA Operations of sample receipt; the federal laboratory performs the test; and the federal laboratory shares the results with the Area/Regional CFIA Operations, who then forwards the result to the P/T/M Inspector (originator of sample).
For suspected sporadic listeriosis cases, food samples may be directed to the Listeriosis Reference Service.
*For suspected sporadic listeriosis cases, food samples may be directed to the Listeriosis Reference Centre
Appendix B: Sample Collection Form
1 – Submitter
Courier Code
Clinician / Health Inspector / Surname
E-mail:
Tel:
Fax:
Date and Time of Collection:
YYYY/MM/DD
HH : MM
2 – Case Information
No.
Sex F/M/U
Date of Birth yyyy/mm/dd
Last Name of Individual Associated (optional)
First Name (optional)
Postal Code Submitter Lab No.
Public Health Unit Case / Outbreak No.
Date of Symptoms Onset: YYYY/MM/DD
Time of Symptoms Onset: HH : MM
Fever
Chills
Headache/Stiff Neck
Diarrhea
Cramps
Encephalitis
meningitis
Watery
Rash
Respiratory Symptoms
Other (specify)
Recent Travel (specify)
3 – Location Information
Place of Eating:
Home
Restaurant
Other
Name:
Address:
Postal Code:
Date and Time of Eating:
YYYY/MM/DD - HH : MM
Place of Food Preparation / Manufacturing
Name:
Address:
Postal Code:
Brand Name
Common Name
Unit Size (e.g., 50g or 125 ml, etc.)
Best Before / Expiry / Use by Date
Packed on Date / Manufacturing Date
Container Type (vacuum pack, rigid plastic, etc.)
Storage Information
Opened or Unopened
Lot Number(s)
Universal Product Code (UPC)
Number of Samples / Sub-samples Taken
Shelf Life of Product (if possible)
Label Claims / Preparation or Serving Instruction
4 – Reason for Test
Purpose of Collection
Clinical Illness Investigation
Other (specify)
Tests Requested
Confirmed Etiological Agent(s)
Specimen Type and Site
Suspect Food
Follow-up sample
Left over
Other (specify)
Same batch / control
Comments:
Insufficient sample for testing
Unsuitable for testing
Final report
Further report to follow
Date Received: YYYY/MM/DD
Examined By:
Date Reported: YYYY/MM/DD
Checked By:
Appendix C
CFIA Food Safety Science Directorate (FSSD)
Laboratory Coordination Division (LCD)
Please use LCD email account for contact: CFIA.LCD-DCL.ACIA@inspection.gc.ca
This account is monitored and a subject matter expert will respond.
Annex 14
Multi-jurisdictional Enteric Illness Outbreak investigations linked to contact with animals or animal Foods
Definitions
The following definitions are provided to establish a common understanding of the terms in this document.
Enteric Zoonoses: A disease of the gastrointestinal tract caused by an infection resulting from the ingestion of bacteria, viruses, or parasites that is transmitted between humans and animals.
Introduction
Globalization has impacted the sale and distribution of animals and animal foods and many recent national and international animal-related enteric illness outbreaks have been linked to chicks, reptiles and amphibians, rodents and also pet food and treats. These types of outbreaks can pose unique challenges in that the regulations and responsibilities around animals and animal food as a source of enteric illness in people are less comprehensive and clear than for food safety. Enteric illness outbreaks linked to animals are typically detected and investigated through the same enteric illness networks and infrastructure as for foodborne outbreaks. It therefore follows, that the principles and mechanisms of the FIORP can be applied in these situations, with some key differences noted.
Purpose
The purpose of this Annex is to provide further details, clarity and notable exceptions to the FIORP roles and responsibilities and operating procedures to be used when multi-jurisdictional enteric illness outbreaks are linked, or suspected to be linked, to animals or their foods.
Roles and responsibilities
Responsibilities for responding to enteric illness outbreaks linked to contact with animals or animal foods are also shared between F/P/T and local/regional jurisdictions. The response to such situations involves collaboration and cooperation among all those involved, and will vary by the type of animal or animal food.
Provincial and Territorial Authorities
Local/regional health officials in individual P/Ts generally have the mandate under P/T disease control legislation to investigate human illness outbreaks that occur within their boundaries, including those with a zoonotic source, and regardless of the type of animal or animal food. Regional P/T public health officials conduct enteric illness surveillance and may also carry out inspection and education activities to reduce the risk of enteric illnesses.
Depending on the P/T, other departments (e.g. agriculture, environment/natural resources) may also have a role in animal-related enteric illness investigations. The role of P/T agriculture and environment agencies on issues related to domestic and wild animal health may include support in the field investigation, traceback, testing and management activities, if applicable. Most Chief Veterinary Officers or veterinary designates have a mandate to provide support for zoonotic disease investigations; the nature and extent of that support will vary depending on the pathogen, the type of animal or animal involved, the public health importance or burden of the issue, and resource availability. All of the provinces have laws in place that enable officials to respond to disease outbreaks or situations that are a public health concern. Some P/Ts have specific animal health legislation for select enteric zoonotic pathogens and may also carry out related inspection and education activities at the P/T level.
Additionally, some P/Ts have their own foodborne or zoonotic outbreak response protocols that may apply in these instances, to guide the collaborative response within the P/T. Local/regional or P/T officials may also, in some cases, request the assistance of HC, PHAC, or the CFIA in the response to a potential enteric illness outbreak with a zoonotic source.
Federal Authorities
Under the federal Minister of Health, PHAC, HC, and the CFIA have limited legislated responsibilities for responding to enteric illness events linked to animals or animal foods.
Public Health Agency of Canada
PHAC provides coordination and leadership during multi-jurisdictional outbreak response, regardless of the source, and the roles and responsibilities noted in the FIORP also apply in animal-related outbreaks.
The Centre for Foodborne, Environmental and Zoonotic Infectious Diseases (CFEZID) at PHAC remains the usual first point of contact for notification by P/T and international partners of issues related to actual or potential enteric zoonotic outbreaks. OMD coordinates multi-jurisdictional foodborne illness outbreaks with technical support from the Zoonoses Division (ZD), CFEZID. In some instances when there is not a federal or P/T animal authority providing assistances (Agriculture/Environment), CFEZID may also support limited traceback investigation. CFEZID may also provide consultation and content expertise for P/T investigations upon request.
The National Microbiology Laboratory (NML) provides reference services for strain identification and characterization, national laboratory-based surveillance, and dissemination of information through PulseNet Canada and the National Enteric Surveillance Program (NESP), as previously described. The NML also provides laboratory reference services to local, P/T and national investigations upon request for animal-related isolates, including Salmonella serotype identification and characterization by PFGE. Provincial laboratories involved in the culturing of animal related samples (e.g. feces, pet food, environmental drag swabs) are able to forward isolates to NML-Guelph for this service.
Canadian Food Inspection Agency
The CFIA delivers federal inspection and enforcement services related to animals under the Health of Animals Act, legislation dealing with diseases and toxic substances that may affect animals or that may be transmitted by animals to persons and the protection of animals. The activities are restricted to animal reportable diseases and do not include enteric pathogens such as verotoxigenic E. coli and Salmonella. CFIA provides some oversight in federally-licensed hatcheries, under the Hatchery Regulations, and provides support during public health investigations linked to chicks from federally-licensed hatcheries. The CFIA also regulates the importation of pet food and related products in order to prevent the introduction of foreign animal disease which could pose a risk to the health of Canadian livestock and provides verification and certification services for pet foods that are made in Canada and intended for export. Domestically manufactured pet food for sale in Canada is not regulated by CFIA.
Despite a limited regulatory role for enteric illness outbreaks linked to animals and animal foods, the CFIA may provide content expertise in traceback, testing and identifying appropriate risk management options. Requests for CFIA assistance in the investigation and control of animal-related enteric illness outbreaks are reviewed as they are received, and engagement will depend on the type of issue including its scale and seriousness, along with the type of assistance requested. Depending on the P/T, traceback and field investigations can be the responsibility of local/provincial/territorial public health authorities or P/T agriculture.
Groups within the CFIA that may play a role in an investigation include:
- Regional inspection staff, including Area Recall Coordinators (ARCs), are the usual first point of contact within the CFIA for local/regional health units and may assist in collecting and submitting samples for testing on a limited basis, although this is not routine practice.
- The Import/Export Animal Products and By-Products Section, of the Animal Import/Export Division, Animal Health Directorate may be involved if the product under investigation is imported or exported.
- The Hatchery Programs (Policy and Programs Branch and Operations Branch) support investigations linked to chicks originating from a federally-licensed hatchery.
- The Office of the Chief Veterinary Officer for Canada may provide strategic policy advice.
Health Canada
In rare cases, HC may be involved or assist with the investigation and management of human enteric illness outbreaks specifically linked to pet food and treats as follows:
The Consumer Product Safety Directorate (CPSD) of the Healthy Environments and Consumer Safety (HECS) Branch of Health Canada identifies, assesses, manages and communicates health or safety risks to Canadians, associated with consumer products. Some commercially sold prepared pet foods and treats are considered consumer products under the Canada Consumer Product Safety Act (CCPSA), provided that they meet the definition of a consumer product and are not excluded under section 4 or schedule 1 of the Act. When contaminated pet food or treats are identified as the likely source of human illness during an outbreak investigation, CPSD may be contacted to determine if the implicated product falls under the authority of the CCPSA. If the contaminated product is determined to be a consumer product that poses a human health risk, CPSD will work in partnership with industry (manufacturers, retailers and distributors) to facilitate the removal of the implicated product from the marketplace, and will post product advisories, warnings or recalls as required.
Other agencies, organizations and associations
Expertise from other agencies, organizations and associations may be sought to assist in the investigation, control and prevention of outbreaks caused by contact with animals or animal foods, including the Canadian Border Services Agency, Canadian Veterinary Medical Association, and industry (livestock and poultry) representatives such as the Pet Industry Joint Advisory Council of Canada.
Operating procedures
In general, the principles and operating procedures used in detecting and investigating foodborne illness outbreaks are also applicable if the suspect source is related to animal exposure; however as noted above, the partners comprising the investigative team will be different and depend on the situation. There are some operational differences worth noting which are described in the remainder of the document. All other sections in the FIORP not specifically referenced here apply directly.
Notification of partners
Similar to a potential foodborne outbreak, Public Health Alerts (CNPHI) can be used as a means for early notification of public health partners. Notifications are typically posted under the Enteric, Food, and Waterborne disease module, however cross posting on the Non-enteric Zoonotic disease module may also be considered to reach a different public health audience. Depending on the nature of the event, notification of federal and P/T animal health partners, who do not receive CNPHI alerts, should also be considered. An Enteric Zoonoses contact list of federal and P/T partners is maintained by PHAC and updated on a quarterly basis.
OICC activation
When an OICC is activated, a notification is sent to the FIORP Duty Officers and P/T epidemiology and laboratory representatives. Recognizing that the FIORP Duty Officers have the responsibility to ensure senior officials within their organization are briefed, notification for animal-related OICC activations should be accompanied with a reminder to forward the notification to relevant parties within their jurisdiction, which may vary from foodborne outbreaks.
Composition of the OICC
An OICC established to investigate animal-related outbreaks should similarly be composed of representatives with the authority to make decisions related to technical and operational issues and have access to senior decision-makers for issues with policy implications. The composition of the OICC will depend on the nature of the outbreak, but will typically include epidemiological, laboratory, animal health and communication experts.
Coordinated investigations
Epidemiological investigations
As with foodborne outbreak investigations, the epidemiological investigation for animal-related outbreaks will be conducted similarly. Efforts will be made to standardize data collection early in the investigation, often with the animal-focused questionnaire specific to the situation. Consideration should be given to collecting human and animal data in a coordinated way to allow for future data integration if needed.
Laboratory investigations
Both epidemiological and animal health investigations usually involve laboratory testing. The process for testing is generally similar for animal-related investigations; however P/T capacity for testing animal health samples varies. In some cases a partner may not have the necessary capacity or expertise to perform the necessary test(s). This should be raised at OICC meetings to ensure samples can be sent to the appropriate lab with the required expertise and capacity.
Animal health investigations
When the source of an outbreak is suspected to be an animal or related products (e.g. pet food), an investigation should be conducted to determine whether the animal/product may be responsible for the outbreak and to strive to identify the root cause of the contamination.
Many reportable human enteric illness pathogens are not reportable in animal populations, so the role of P/T and federal agriculture authorities in supporting the animal health investigation may vary. In general, public health authorities remain the primary lead on the animal health investigation, with support from animal health colleagues. The following represent past outbreak investigation scenarios with a brief description of lead and supporting roles. It is important to note that future investigations may result in different roles or division of responsibilities depending on the jurisdictions of the partners involved.
| Outbreak source | Description of animal health investigation |
|---|---|
| Pet food/ treats | Led by OICC lead; no national animal health lead. Traceback was conducted primarily by public health authorities. Samples were collected by public health and CFIA. Testing was conducted at provincial agriculture and CFIA labs with support for serotyping and genetic testing at NML. |
| Live poultry (chicks) | Led by OICC lead; no national animal health lead. Investigation at the source hatchery was led by provincial agriculture authority where the hatchery was located. Traceback was conducted jointly between public health and animal health authorities. Sampling was conducted by agriculture/environment in multiple provinces. Testing of animal samples was conducted at provincial agriculture labs with support for serotyping and genetic testing at the NML. |
| Reptiles or amphibians | Led by OICC lead; no national animal health lead. Traceback and sampling was conducted by public health authorities, with sampling by agriculture in one province. Testing of animal samples was conducted at provincial public health and agriculture labs with support for serotyping and genetic testing at the NML. |
| Feeder rodents | Led by OICC lead; no national animal health lead. Traceback and sampling was conducted by public health authorities, with sampling by agriculture in one province. Testing of animal samples was conducted at provincial agriculture lab with support for serotyping and genetic testing at the NML. |
Public health actions
Actions undertaken during a zoonotic enteric illness outbreak to address the source of the outbreak and prevent further cases of human illness may include a wide range of activities by one or more of the partners. Examples include:
- Public communication outlining recommended prevention and control activities;
- For pet food and treats, recalling, detaining, or disposing of a contaminated food product;
- Inspection, closure, sanitation, and review of practices at implicated facilities such as hatcheries and poultry farms; and
- Case and contact management.
Each partner will conduct the necessary mitigation actions under its respective mandate. The OICC coordinates information sharing related to these actions and facilitates discussions concerning the timing of these actions.
Exchange of industry information
During an investigation, all implicated companies/organizations should be kept informed of developments. If a lead investigator has been identified (e.g. P/T agriculture in hatchery-implicated human illness,) they would be the primary contact. However, in instances where there is no responsible agriculture authority identified, the OICC lead may be the primary contact unless otherwise agreed.